Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques
- PMID: 19525965
- PMCID: PMC4334439
- DOI: 10.1038/nm.1974
Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques
Abstract
Neutralizing antibodies are thought to be crucial for HIV vaccine protection, but studies in animal models suggest that high antibody concentrations are required. This is a major potential hurdle for vaccine design. However, these studies typically apply a large virus inoculum to ensure infection in control animals in single-challenge experiments. In contrast, most human infection via sexual encounter probably involves repeated exposures to much lower doses of virus. Therefore, animal studies may have provided an overestimate of the levels of antibodies required for protection in humans. We investigated whether plasma concentrations of antibody corresponding to relatively modest neutralization titers in vitro could protect macaques from repeated intravaginal exposure to low doses of a simian immunodeficiency virus-HIV chimera (SHIV) that uses the CC chemokine receptor 5 (CCR5) co-receptor. An effector function-deficient variant of the neutralizing antibody was also included. The results show that a substantially larger number of challenges is required to infect macaques treated with neutralizing antibody than control antibody-treated macaques, and support the notion that effector function may contribute to antibody protection. Overall, the results imply that lower amounts of antibody than previously considered protective may provide benefit in the context of typical human exposure to HIV-1.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

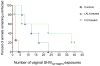
Comment in
-
Blocking and tackling HIV.Nat Med. 2009 Aug;15(8):841-2. doi: 10.1038/nm0809-841. Nat Med. 2009. PMID: 19661984 No abstract available.
References
-
- Mascola JR. Defining the protective antibody response for HIV-1. Curr Mol Med. 2003;3:209–216. - PubMed
-
- Wawer MJ, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. - PubMed
-
- Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. - PubMed
-
- Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. - PubMed
-
- Gauduin MC, et al. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

