Structural basis for ESCRT-III protein autoinhibition
- PMID: 19525971
- PMCID: PMC2712734
- DOI: 10.1038/nsmb.1621
Structural basis for ESCRT-III protein autoinhibition
Abstract
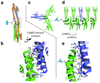
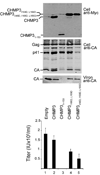
Endosomal sorting complexes required for transport-III (ESCRT-III) subunits cycle between two states: soluble monomers and higher-order assemblies that bind and remodel membranes during endosomal vesicle formation, midbody abscission and enveloped virus budding. Here we show that the N-terminal core domains of increased sodium tolerance-1 (IST1) and charged multivesicular body protein-3 (CHMP3) form equivalent four-helix bundles, revealing that IST1 is a previously unrecognized ESCRT-III family member. IST1 and its ESCRT-III binding partner, CHMP1B, both form higher-order helical structures in vitro, and IST1-CHMP1 interactions are required for abscission. The IST1 and CHMP3 structures also reveal that equivalent downstream alpha5 helices can fold back against the core domains. Mutations within the CHMP3 core-alpha5 interface stimulate the protein's in vitro assembly and HIV-inhibition activities, indicating that dissociation of the autoinhibitory alpha5 helix from the core activates ESCRT-III proteins for assembly at membranes.
Figures






References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

