EGFR juxtamembrane domain, membranes, and calmodulin: kinetics of their interaction
- PMID: 19527647
- PMCID: PMC2712057
- DOI: 10.1016/j.bpj.2009.03.027
EGFR juxtamembrane domain, membranes, and calmodulin: kinetics of their interaction
Abstract
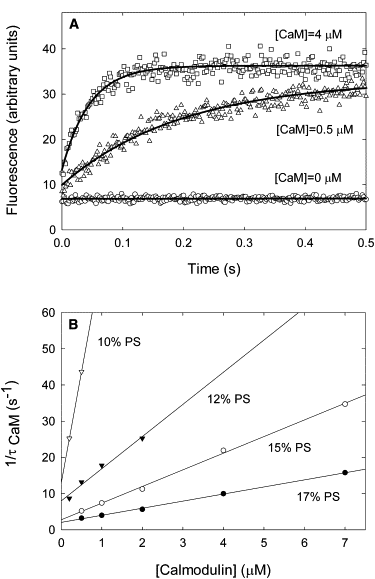
Calcium/calmodulin (Ca/CaM) binds to the intracellular juxtamembrane domain (JMD) of the epidermal growth factor receptor (EGFR). The basic JMD also binds to acidic lipids in the inner leaflet of the plasma membrane, and this interaction may contribute an extra level of autoinhibition to the receptor. Binding of a ligand to the EGFR produces a rapid increase in intracellular calcium, [Ca2+]i, and thus Ca/CaM. How does Ca/CaM compete with the plasma membrane for the JMD? Does Ca/CaM directly pull the JMD off the membrane or does Ca/CaM only bind to the JMD after it has dissociated spontaneously from the bilayer? To answer this question, we studied the effect of Ca/CaM on the rate of dissociation of fluorescent JMD peptides from phospholipid vesicles by making kinetic stop-flow measurements. Ca/CaM increases the rate of dissociation: an analysis of the differential equations that describe the dissociation shows that Ca/CaM must directly pull the basic JMD peptide off the membrane surface. These measurements lead to a detailed atomic-level mechanism for EGFR activation that reconciles the existence of preformed EGFR dimers/oligomers with the Kuriyan allosteric model for activation of the EGFR kinase domains.
Figures



References
-
- Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. - PubMed
-
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. - PubMed
-
- Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. - PubMed
-
- Jorissen R.N., Walker F., Pouliot N., Garrett T.P.J., Ward C.W. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 2003;284:31–53. - PubMed
-
- Citri A., Yarden Y. EGF-ErbB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 2006;7:505–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

