Pin-hole array correlation imaging: highly parallel fluorescence correlation spectroscopy
- PMID: 19527665
- PMCID: PMC2712041
- DOI: 10.1016/j.bpj.2009.03.023
Pin-hole array correlation imaging: highly parallel fluorescence correlation spectroscopy
Abstract
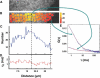
In this work, we describe pin-hole array correlation imaging, a multipoint version of fluorescence correlation spectroscopy, based upon a stationary Nipkow disk and a high-speed electron multiplying charged coupled detector. We characterize the system and test its performance on a variety of samples, including 40 nm colloids, a fluorescent protein complex, a membrane dye, and a fluorescence fusion protein. Our results demonstrate that pin-hole array correlation imaging is capable of simultaneously performing tens or hundreds of fluorescence correlation spectroscopy-style measurements in cells, with sufficient sensitivity and temporal resolution to study the behaviors of membrane-bound and soluble molecules labeled with conventional chemical dyes or fluorescent proteins.
Figures








References
-
- Kim S.A., Heinze K.G., Schwille P. Fluorescence correlation spectroscopy in living cells. Nat. Methods. 2007;4:963–973. - PubMed
-
- Krichevsky O., Bonnet G. Fluorescence correlation spectroscopy: the technique and its applications. Rep. Prog. Phys. 2002;65:251–297.
-
- Brinkmeier M., Dorre K., Stephan J., Eigen M. Two-beam cross-correlation: a method to characterize transport phenomena in micrometer-sized structures. Anal. Chem. 1999;71:609–616. - PubMed
-
- Dittrich P.S., Schwille P. Spatial two-photon fluorescence cross-correlation spectroscopy for controlling molecular transport in microfluidic structures. Anal. Chem. 2002;74:4472–4479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

