Topography and nanomechanics of live neuronal growth cones analyzed by atomic force microscopy
- PMID: 19527666
- PMCID: PMC2712036
- DOI: 10.1016/j.bpj.2009.03.032
Topography and nanomechanics of live neuronal growth cones analyzed by atomic force microscopy
Abstract
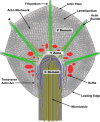
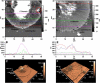
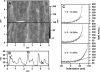
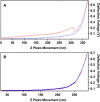
Neuronal growth cones are motile structures located at the end of axons that translate extracellular guidance information into directional movements. Despite the important role of growth cones in neuronal development and regeneration, relatively little is known about the topography and mechanical properties of distinct subcellular growth cone regions under live conditions. In this study, we used the AFM to study the P domain, T zone, and C domain of live Aplysia growth cones. The average height of these regions was calculated from contact mode AFM images to be 183 +/- 33, 690 +/- 274, and 1322 +/- 164 nm, respectively. These findings are consistent with data derived from dynamic mode images of live and contact mode images of fixed growth cones. Nano-indentation measurements indicate that the elastic moduli of the C domain and T zone ruffling region ranged between 3-7 and 7-23 kPa, respectively. The range of the measured elastic modulus of the P domain was 10-40 kPa. High resolution images of the P domain suggest its relatively high elastic modulus results from a dense meshwork of actin filaments in lamellipodia and from actin bundles in the filopodia. The increased mechanical stiffness of the P and T domains is likely important to support and transduce tension that develops during growth cone steering.
Figures





Similar articles
-
High-resolution analysis of neuronal growth cone morphology by comparative atomic force and optical microscopy.J Neurobiol. 2006 Dec;66(14):1529-43. doi: 10.1002/neu.20318. J Neurobiol. 2006. PMID: 17058186
-
The Microtubule-Associated Protein Tau Mediates the Organization of Microtubules and Their Dynamic Exploration of Actin-Rich Lamellipodia and Filopodia of Cortical Growth Cones.J Neurosci. 2018 Jan 10;38(2):291-307. doi: 10.1523/JNEUROSCI.2281-17.2017. Epub 2017 Nov 22. J Neurosci. 2018. PMID: 29167405 Free PMC article.
-
Cortactin colocalizes with filopodial actin and accumulates at IgCAM adhesion sites in Aplysia growth cones.J Neurosci Res. 2009 Apr;87(5):1057-68. doi: 10.1002/jnr.21937. J Neurosci Res. 2009. PMID: 19021290 Free PMC article.
-
Molecular mechanism of actin-dependent retrograde flow in lamellipodia of motile cells.Front Biosci. 1997 Jun 1;2:d260-70. doi: 10.2741/a189. Front Biosci. 1997. PMID: 9206973 Review.
-
Nanomechanics of Cells and Biomaterials Studied by Atomic Force Microscopy.Adv Healthc Mater. 2015 Nov 18;4(16):2456-74. doi: 10.1002/adhm.201500229. Epub 2015 Jul 22. Adv Healthc Mater. 2015. PMID: 26200464 Review.
Cited by
-
Mechanotransduction in neuronal cell development and functioning.Biophys Rev. 2019 Oct;11(5):701-720. doi: 10.1007/s12551-019-00587-2. Epub 2019 Oct 15. Biophys Rev. 2019. PMID: 31617079 Free PMC article. Review.
-
Quantifying mechanical force in axonal growth and guidance.Front Cell Neurosci. 2015 Sep 16;9:359. doi: 10.3389/fncel.2015.00359. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26441530 Free PMC article. Review.
-
Biophysics of substrate interaction: influence on neural motility, differentiation, and repair.Dev Neurobiol. 2011 Nov;71(11):1090-101. doi: 10.1002/dneu.20947. Dev Neurobiol. 2011. PMID: 21739614 Free PMC article. Review.
-
Measuring the elastic properties of living cells through the analysis of current-displacement curves in scanning ion conductance microscopy.Pflugers Arch. 2012 Sep;464(3):307-16. doi: 10.1007/s00424-012-1127-6. Epub 2012 Jun 29. Pflugers Arch. 2012. PMID: 22744227
-
Early T-cell activation biophysics.HFSP J. 2009 Dec;3(6):401-11. doi: 10.2976/1.3254098. Epub 2009 Nov 10. HFSP J. 2009. PMID: 20514131 Free PMC article.
References
-
- Charron F., Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. - PubMed
-
- Dickson B.J. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. - PubMed
-
- Song H., Poo M. The cell biology of neuronal navigation. Nat. Cell Biol. 2001;3:E81–E88. - PubMed
-
- Huber A.B., Kolodkin A.L., Ginty D.D., Cloutier J.F. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 2003;26:509–563. - PubMed
-
- Dent E.W., Gertler F.B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

