Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin
- PMID: 19527669
- PMCID: PMC2712047
- DOI: 10.1016/j.bpj.2009.03.046
Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin
Abstract
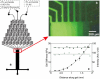
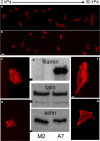
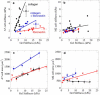
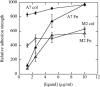
Cell types from many tissues respond to changes in substrate stiffness by actively remodeling their cytoskeletons to alter spread area or adhesion strength, and in some cases changing their own stiffness to match that of their substrate. These cell responses to substrate stiffness are linked to substrate-induced changes in the state, localization, and amount of numerous proteins, but detailed evidence for the requirement of specific proteins in these distinct forms of mechanical response are scarce. Here we use microfluidics techniques to produce gels with a gradient of stiffness to show the essential function of filamin A in cell responses to mechanical stimuli and dissociate cell spreading and stiffening by contrasting responses of a pair of human melanoma-derived cell lines that differ in expression of this actin cross-linking protein. M2 melanoma cells null for filamin A do not alter their adherent area in response to increased substrate stiffness when they link to the substrate only through collagen receptors, but change adherent area normally when bound through fibronectin receptors. In contrast, filamin A-replete A7 cells change adherent area on both substrates and respond more strongly to collagen I-coated gels than to fibronectin-coated gels. Strikingly, A7 cells alter their stiffness, as measured by atomic force microscopy, to match the elastic modulus of the substrate immediately adjacent to them on the gradient. M2 cells, in contrast, maintain a constant stiffness on all substrates that is as low as that of A7 cells on the softest gels examined (1000 Pa). Comparison of cell spreading and cell stiffening on the same gradient substrates shows that cell spreading is uncoupled from stiffening. At saturating collagen and fibronectin concentrations, adhesion of M2 cells is reduced compared to that of A7 cells to an extent approximately equal to the difference in adherent area. Filamin A appears to be essential for cell stiffening on collagen, but not for cell spreading on fibronectin. These results have implications for different models of cell protrusion and adhesion and identify a key role for filamin A in altering cellular stiffness that cannot be compensated for by other actin cross-linkers in vivo.
Figures
Similar articles
-
Filamin A is essential for active cell stiffening but not passive stiffening under external force.Biophys J. 2009 May 20;96(10):4326-35. doi: 10.1016/j.bpj.2009.02.035. Biophys J. 2009. PMID: 19450503 Free PMC article.
-
Reprogramming cellular phenotype by soft collagen gels.Soft Matter. 2014 Nov 28;10(44):8829-37. doi: 10.1039/c4sm01602e. Soft Matter. 2014. PMID: 25284029 Free PMC article.
-
Filamin A is required for vimentin-mediated cell adhesion and spreading.Am J Physiol Cell Physiol. 2010 Feb;298(2):C221-36. doi: 10.1152/ajpcell.00323.2009. Epub 2009 Sep 23. Am J Physiol Cell Physiol. 2010. PMID: 19776392 Free PMC article.
-
Mechanical response of single filamin A (ABP-280) molecules and its role in the actin cytoskeleton.J Muscle Res Cell Motil. 2002;23(5-6):525-34. doi: 10.1023/a:1023418725001. J Muscle Res Cell Motil. 2002. PMID: 12785102 Review.
-
Structural portrait of filamin interaction mechanisms.Curr Protein Pept Sci. 2010 Nov;11(7):639-50. doi: 10.2174/138920310794109111. Curr Protein Pept Sci. 2010. PMID: 20887254 Review.
Cited by
-
Attenuation of cell mechanosensitivity in colon cancer cells during in vitro metastasis.PLoS One. 2012;7(11):e50443. doi: 10.1371/journal.pone.0050443. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226284 Free PMC article.
-
Finding the weakest link: exploring integrin-mediated mechanical molecular pathways.J Cell Sci. 2012 Jul 1;125(Pt 13):3025-38. doi: 10.1242/jcs.095794. Epub 2012 Jul 13. J Cell Sci. 2012. PMID: 22797926 Free PMC article. Review.
-
Embryonic stem cells do not stiffen on rigid substrates.Biophys J. 2010 Jul 21;99(2):L19-21. doi: 10.1016/j.bpj.2010.04.057. Biophys J. 2010. PMID: 20643049 Free PMC article.
-
From tissue mechanics to transcription factors.Differentiation. 2013 Oct;86(3):112-20. doi: 10.1016/j.diff.2013.07.004. Epub 2013 Aug 20. Differentiation. 2013. PMID: 23969122 Free PMC article. Review.
-
Dynamic traction force in trabecular meshwork cells: A 2D culture model for normal and glaucomatous states.Acta Biomater. 2024 Feb;175:138-156. doi: 10.1016/j.actbio.2023.12.033. Epub 2023 Dec 25. Acta Biomater. 2024. PMID: 38151067 Free PMC article.
References
-
- Discher D.E., Janmey P., Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
-
- Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. - PubMed
-
- Georges P.C., Janmey P.A. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 2005;98:1547–1553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

