Calcium-dependent signaling and kinases in apicomplexan parasites
- PMID: 19527888
- PMCID: PMC2718762
- DOI: 10.1016/j.chom.2009.05.017
Calcium-dependent signaling and kinases in apicomplexan parasites
Abstract
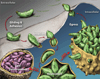
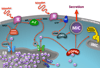
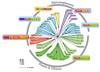
Calcium controls many critical events in the complex life cycles of apicomplexan parasites including protein secretion, motility, and development. Calcium levels are normally tightly regulated and rapid release of calcium into the cytosol activates a family of calcium-dependent protein kinases (CDPKs), which are normally characteristic of plants. CDPKs present in apicomplexans have acquired a number of unique domain structures likely reflecting their diverse functions. Calcium regulation in parasites is closely linked to signaling by cyclic nucleotides and their associated kinases. This Review summarizes the pivotal roles that calcium- and cyclic nucleotide-dependent kinases play in unique aspects of parasite biology.
Figures

References
-
- Alano P, Billker O. Gametocytes and Gametes Chapter 10. In: Sherman IW, editor. Molecular Approaches to Malaria. Washington: ASM Press; 2005.
-
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frishknecht F, Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 2006;12:220–224. - PubMed
-
- Armstrong CM, Goldberg DE. A FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat. Methods. 2007;4:1007–1009. - PubMed
-
- Baker DA, Deng W. Cyclic GMP-dependent protein kinases in protozoa. Front. Biosc. 2005;10:1229–1238. - PubMed
-
- Baker DA, Kelly JM. Purine nucleotide cyclases in the malaria parasite. Trends Parasitol. 2004;20:227–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

