Loa loa Microfilariae evade complement attack in vivo by acquiring regulatory proteins from host plasma
- PMID: 19528206
- PMCID: PMC2738024
- DOI: 10.1128/IAI.01583-08
Loa loa Microfilariae evade complement attack in vivo by acquiring regulatory proteins from host plasma
Abstract
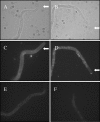
Loa loa is a filarial nematode that infects humans. The adults live in subcutaneous tissues and produce microfilariae that live for several weeks in the blood circulation in order to be transmitted to another person via blood meals of a dipterian vector. As microfilariae live in continuous contact with plasma, it is obvious that they evade the complement system. We studied markers of complement activation and signs of complement regulation on Loa loa microfilariae in vivo. The microfilariae were isolated from anticoagulated blood samples of a Loa loa-infected Caucasian patient. C1q and some mannose-binding lectin but only a limited amount of C3b or C4b fragments and practically no C5 or C5b-9 were present on the microfilariae. The covalently microfilaria-bound C3 and C4 depositions were mainly inactive iC3b, C3c, and iC4b fragments indicating that microfilariae had regulated complement activation in vivo. Also, in vitro deposition of C3b onto the microfilariae upon serum exposure was limited. The patient-isolated microfilariae were found to carry the host complement regulators factor H and C4b-binding protein on the outermost layer, so called sheath. The microfilaria-bound factor H was functionally active. Binding of the complement regulators to the microfilariae was confirmed in vitro using (125)I-labeled factor H and C4b-binding protein. In conclusion, our study shows that Loa loa microfilariae block complement activation and acquire the host complement regulators factor H and C4b-binding protein in blood circulation. This is the first time that binding of complement regulators onto nonviral pathogens has been demonstrated to occur in humans in vivo.
Figures





References
-
- Ajuh, P. M., J. P. Akue, P. Boutin, S. Everaere, and T. G. Egwang. 1995. Loa loa: structural diversity of a 15-kDa repetitive antigen. Exp. Parasitol. 81145-153. - PubMed
-
- Berggård, K., E. Johnsson, E Morfeldt, J. Persson, M. Stålhammar-Carlemalm, and G. Lindahl. 2001. Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Mol. Microbiol. 42539-551. - PubMed
-
- Chandrashekar, R., U. R. Rao, G. R. Rajasekariah, and D. Subrahmanyam. 1984. Separation of viable microfilariae free of blood cells on Percoll gradients. J. Helminthol. 5869-70. - PubMed
-
- Cooper, N. R. 1975. Enzymatic activity of the second component of complement. Biochemistry 144245-4251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

