Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication
- PMID: 19528215
- PMCID: PMC2737996
- DOI: 10.1128/IAI.00438-09
Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication
Abstract
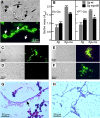
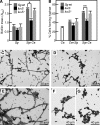
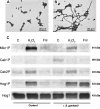
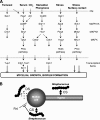
The fungus Candida albicans colonizes human oral cavity surfaces in conjunction with a complex microflora. C. albicans SC5314 formed biofilms on saliva-coated surfaces that in early stages of development consisted of approximately 30% hyphal forms. In mixed biofilms with the oral bacterium Streptococcus gordonii DL1, hyphal development by C. albicans was enhanced so that biofilms consisted of approximately 60% hyphal forms. Cell-cell contact between S. gordonii and C. albicans involved Streptococcus cell wall-anchored proteins SspA and SspB (antigen I/II family polypeptides). Repression of C. albicans hyphal filament and biofilm production by the quorum-sensing molecule farnesol was relieved by S. gordonii. The ability of a luxS mutant of S. gordonii deficient in production of autoinducer 2 to induce C. albicans hyphal formation was reduced, and this mutant suppressed farnesol inhibition of hyphal formation less effectively. Coincubation of the two microbial species led to activation of C. albicans mitogen-activated protein kinase Cek1p, inhibition of Mkc1p activation by H(2)O(2), and enhanced activation of Hog1p by farnesol, which were direct effects of streptococci on morphogenetic signaling. These results suggest that interactions between C. albicans and S. gordonii involve physical (adherence) and chemical (diffusible) signals that influence the development of biofilm communities. Thus, bacteria may play a significant role in modulating Candida carriage and infection processes in the oral cavity.
Figures

References
-
- Baillie, G. S., and L. J. Douglas. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48671-679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

