Novel chimpanzee/human monoclonal antibodies that neutralize anthrax lethal factor, and evidence for possible synergy with anti-protective antigen antibody
- PMID: 19528217
- PMCID: PMC2738033
- DOI: 10.1128/IAI.00200-09
Novel chimpanzee/human monoclonal antibodies that neutralize anthrax lethal factor, and evidence for possible synergy with anti-protective antigen antibody
Abstract
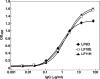
Three chimpanzee Fabs reactive with lethal factor (LF) of anthrax toxin were isolated and converted into complete monoclonal antibodies (MAbs) with human gamma1 heavy-chain constant regions. In a macrophage toxicity assay, two of the MAbs, LF10E and LF11H, neutralized lethal toxin (LT), a complex of LF and anthrax protective antigen (PA). LF10E has the highest reported affinity for a neutralizing MAb against LF (dissociation constant of 0.69 nM). This antibody also efficiently neutralized LT in vitro, with a 50% effective concentration (EC(50)) of 0.1 nM, and provided 100% protection of rats against toxin challenge with a 0.5 submolar ratio relative to LT. LF11H, on the other hand, had a slightly lower binding affinity to LF (dissociation constant of 7.4 nM) and poor neutralization of LT in vitro (EC(50) of 400 nM) and offered complete protection in vivo only at an equimolar or higher ratio to toxin. Despite this, LF11H, but not LF10E, provided robust synergistic protection when combined with MAb W1, which neutralizes PA. Epitope mapping and binding assays indicated that both LF10E and LF11H recognize domain I of LF (amino acids 1 to 254). Although domain I is responsible for binding to PA, neither MAb prevented LF from binding to activated PA. Although two unique MAbs could protect against anthrax when used alone, even more efficient and broader protection should be gained by combining them with anti-PA MAbs.
Figures


References
-
- Albrecht, M. T., H. Li, E. D. Williamson, C. S. LeButt, H. C. Flick-Smith, C. P. Quinn, H. Westra, D. Galloway, A. Mateczun, S. Goldman, H. Groen, and L. W. Baillie. 2007. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect. Immun. 755425-5433. - PMC - PubMed
-
- Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, Y. H. Zhou, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, J. Svitel, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2006. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc. Natl. Acad. Sci. USA 1031882-1887. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

