Hypoxia-inducible factor 2 regulates hepatic lipid metabolism
- PMID: 19528226
- PMCID: PMC2725738
- DOI: 10.1128/MCB.00200-09
Hypoxia-inducible factor 2 regulates hepatic lipid metabolism
Abstract
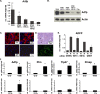
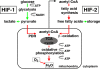
In mammals, the liver integrates nutrient uptake and delivery of carbohydrates and lipids to peripheral tissues to control overall energy balance. Hepatocytes maintain metabolic homeostasis by coordinating gene expression programs in response to dietary and systemic signals. Hepatic tissue oxygenation is an important systemic signal that contributes to normal hepatocyte function as well as disease. Hypoxia-inducible factors 1 and 2 (HIF-1 and HIF-2, respectively) are oxygen-sensitive heterodimeric transcription factors, which act as key mediators of cellular adaptation to low oxygen. Previously, we have shown that HIF-2 plays an important role in both physiologic and pathophysiologic processes in the liver. HIF-2 is essential for normal fetal EPO production and erythropoiesis, while constitutive HIF-2 activity in the adult results in polycythemia and vascular tumorigenesis. Here we report a novel role for HIF-2 in regulating hepatic lipid metabolism. We found that constitutive activation of HIF-2 in the adult results in the development of severe hepatic steatosis associated with impaired fatty acid beta-oxidation, decreased lipogenic gene expression, and increased lipid storage capacity. These findings demonstrate that HIF-2 functions as an important regulator of hepatic lipid metabolism and identify HIF-2 as a potential target for the treatment of fatty liver disease.
Figures
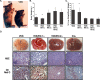

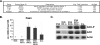
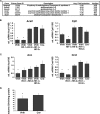
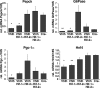
References
-
- Bruder, E. D., P. C. Lee, and H. Raff. 2005. Lipid and fatty acid profiles in the brain, liver, and stomach contents of neonatal rats: effects of hypoxia. Am. J. Physiol. Endocrinol. Metab. 288E314-E320. - PubMed
-
- Brunt, E. M., B. A. Neuschwander-Tetri, D. Oliver, K. R. Wehmeier, and B. R. Bacon. 2004. Nonalcoholic steatohepatitis: histologic features and clinical correlations with 30 blinded biopsy specimens. Hum. Pathol. 351070-1082. - PubMed
-
- Chakravarthy, M. V., Z. Pan, Y. Zhu, K. Tordjman, J. G. Schneider, T. Coleman, J. Turk, and C. F. Semenkovich. 2005. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1309-322. - PubMed
-
- Das, D. K., J. Ayromlooi, and A. Neogi. 1983. Effect of ischemia on fatty acid metabolism in fetal lung. Life Sci. 33569-576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
