Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects
- PMID: 19528280
- PMCID: PMC2737845
- DOI: 10.1128/AAC.00106-09
Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects
Abstract
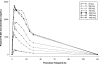
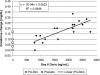
PA-824 is a novel antibacterial agent that has shown in vitro activity against both drug-sensitive and drug-resistant Mycobacterium tuberculosis. The compound's MIC is between 0.015 and 0.25 microg/ml for drug-sensitive strains and between 0.03 and 0.53 microg/ml for drug-resistant strains. In addition, it is active against nonreplicating anaerobic Mycobacterium tuberculosis. The safety, tolerability, and pharmacokinetics of PA-824 were evaluated in two escalating-dose clinical studies, one a single-dose study and the other a multiple-dose study (up to 7 days of daily dosing). In 58 healthy subjects dosed with PA-824 in these studies, the drug candidate was well tolerated, with no significant or serious adverse events. In both studies, following oral administration PA-824 reached maximal plasma levels in 4 to 5 h independently of the dose. Maximal blood levels averaged approximately 3 microg/ml (1,500-mg dose) in the single-dose study and 3.8 microg/ml (600-mg dose) in the multiple-dose study. Steady state was achieved after 5 to 6 days of daily dosing, with an accumulation ratio of approximately 2. The elimination half-life averaged 16 to 20 h. Overall, PA-824 was well tolerated following oral doses once daily for up to 7 days, and pharmacokinetic parameters were consistent with a once-a-day regimen. The results of these studies, combined with the demonstrated activity of PA-824 against drug-sensitive and multidrug-resistant Mycobacterium tuberculosis, support the investigation of this novel compound for the treatment of tuberculosis.
Figures

References
-
- Lenaerts, A. J., V. Gruppo, K. S. Marietta, C. M. Johnson, D. K. Driscoll, N. M. Tompkins, J. D. Rose, R. C. Reynolds, and I. M. Orme. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294-2301. - PMC - PubMed
-
- Manjunatha, U. H., H. Boshoff, C. S. Dowd, L. Zhang, T. J. Albert, J. E. Norton, L. Daniels, T. Dick, S. S. Pang, and C. E. Barry III. 2006. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 103:431-436. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

