Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g
- PMID: 19528297
- PMCID: PMC2711607
- DOI: 10.1083/jcb.200811099
Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g
Abstract
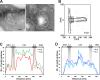
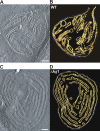
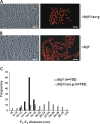
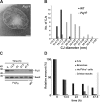
Crista junctions (CJs) are important for mitochondrial organization and function, but the molecular basis of their formation and architecture is obscure. We have identified and characterized a mitochondrial membrane protein in yeast, Fcj1 (formation of CJ protein 1), which is specifically enriched in CJs. Cells lacking Fcj1 lack CJs, exhibit concentric stacks of inner membrane in the mitochondrial matrix, and show increased levels of F(1)F(O)-ATP synthase (F(1)F(O)) supercomplexes. Overexpression of Fcj1 leads to increased CJ formation, branching of cristae, enlargement of CJ diameter, and reduced levels of F(1)F(O) supercomplexes. Impairment of F(1)F(O) oligomer formation by deletion of its subunits e/g (Su e/g) causes CJ diameter enlargement and reduction of cristae tip numbers and promotes cristae branching. Fcj1 and Su e/g genetically interact. We propose a model in which the antagonism between Fcj1 and Su e/g locally modulates the F(1)F(O) oligomeric state, thereby controlling membrane curvature of cristae to generate CJs and cristae tips.
Figures





References
-
- Bornhövd C., Vogel F., Neupert W., Reichert A.S. 2006. Mitochondrial membrane potential is dependent on the oligomeric state of F1F0-ATP synthase supracomplexes.J. Biol. Chem. 281:13990–13998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

