A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes
- PMID: 19528348
- PMCID: PMC2708801
- DOI: 10.2353/ajpath.2009.081002
A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes
Abstract
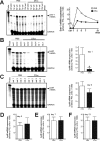
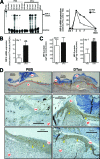
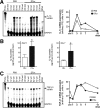
Whether the wound macrophage is a key regulatory inflammatory cell type in skin repair has been a matter of debate. A transgenic mouse model mediating inducible macrophage depletion during skin repair has not been used to date to address this question. Here, we specifically rendered the monocyte/macrophage leukocyte lineage sensitive to diphtheria toxin by expressing the lysozyme M promoter-driven, Cre-mediated excision of a transcriptional STOP cassette from the simian DT receptor gene in mice (lysM-Cre/DTR). Application of diphtheria toxin to lysM-Cre/DTR mice led to a rapid reduction in both skin tissue and wound macrophage numbers at sites of injury. Macrophage-depleted mice revealed a severely impaired wound morphology and delayed healing. In the absence of macrophages, wounds were re-populated by large numbers of neutrophils. Accordingly, macrophage-reduced wound tissues exhibited the increased and prolonged persistence of macrophage inflammatory protein-2, macrophage chemoattractant protein-1, interleukin-1beta, and cyclooxygenase-2, paralleled by unaltered levels of bioactive transforming growth factor-beta1. Altered expression patterns of vascular endothelial growth factor on macrophage reduction were associated with a disturbed neo-vascularization at the wound site. Impaired wounds revealed a loss of myofibroblast differentiation and wound contraction. Our data in the use of lysM-Cre/DTR mice emphasize the pivotal function of wound macrophages in the integration of inflammation and cellular movements at the wound site to enable efficient skin repair.
Figures









References
-
- Peters T, Sindrilaru A, Hinz B, Hinrichs R, Menke A, Al-Azzeh EA, Holzwarth K, Oreshkova T, Wang H, Kess D, Walzog B, Sulyok S, Sunderkötter C, Friedrich W, Wlaschek M, Krieg T, Scharffetter-Kochanek K. Wound healing defect of CD18−/− mice due to a decrease in TGF-β1 and myofibroblast differentiation. EMBO J. 2005;24:3400–3410. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

