Hypoxia-mediated up-regulation of Pim-1 contributes to solid tumor formation
- PMID: 19528349
- PMCID: PMC2708825
- DOI: 10.2353/ajpath.2009.080972
Hypoxia-mediated up-regulation of Pim-1 contributes to solid tumor formation
Abstract
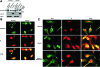
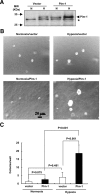
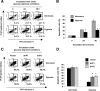
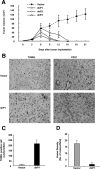
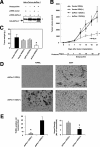
Tumor hypoxia directly promotes genomic instability and facilitates cell survival, resulting in tumors with a more aggressive phenotype. The proto-oncogene pim-1 regulates apoptosis and the cell cycle by phosphorylating target proteins. Overexpression of Pim-1 can cause genomic instability and contribute to lymphomagenesis. It is not clear whether Pim-1 is involved in hypoxia-mediated tumor survival in solid tumors. Here, we show that hypoxia can stabilize Pim-1 by preventing its ubiquitin-mediated proteasomal degradation and can cause Pim-1 translocation from the cytoplasm to the nucleus. Importantly, overexpression of Pim-1 increases NIH3T3 cell transformation exclusively under hypoxic conditions, suggesting that Pim-1 expression under hypoxia may be implicated in the transformation process of solid tumors. Also, blocking Pim-1 function by introduction of dominant negative Pim-1 resensitizes pancreatic cancer cells to apoptosis induced by glucose-deprivation under hypoxia. Introduction of short interfering RNAs for Pim-1 also resensitizes cancer cells to glucose deprivation under hypoxic conditions, while forced overexpression of Pim-1 causes solid tumor cells to become resistant to glucose deprivation. Moreover, dominant negative Pim-1 reduces tumorigenicity in pancreatic cancer cells and HeLa xenograft mouse models. Together, our studies indicate that Pim-1 plays a distinct role in solid tumor formation in vivo, implying that Pim-1 may be a novel target for cancer therapy.
Figures



References
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
-
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. - PubMed
-
- Cuypers HT, Selten G, Berns A, Geurts van Kessel AH. Assignment of the human homologue of Pim-1, a mouse gene implicated in leukemogenesis, to the pter-q12 region of chromosome 6. Hum Genet. 1986;72:262–265. - PubMed
-
- van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T, Berns A. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989;56:673–682. - PubMed
-
- Allen JD, Berns A. Complementation tagging of cooperating oncogenes in knockout mice. Semin Cancer Biol. 1996;7:299–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

