Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial
- PMID: 19528372
- PMCID: PMC4879689
- DOI: 10.1200/JCO.2008.19.6550
Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial
Abstract
Purpose: Decitabine, a DNA-targeted hypomethylating agent, is approved by the United States Food and Drug Administration for treatment of patients with myelodysplastic syndromes (MDS) on a schedule of 15 mg/m(2) administered via intravenous (IV) infusion every 8 hours for 3 days. This study assessed the efficacy and safety of an alternative dosing regimen administered on an outpatient basis in academic and community-based practices.
Patients and methods: Patients were treated with decitabine 20 mg/m(2) by IV infusion daily for 5 consecutive days every 4 weeks. Eligible patients were > or = 18 years of age and had MDS (de novo or secondary) of any French-American-British (FAB) subtype and an International Prognostic Scoring System (IPSS) score > or = 0.5. The primary end point was the overall response rate (ORR) by International Working Group (IWG 2006) criteria; secondary end points included cytogenetic responses, hematologic improvement (HI), response duration, survival, and safety.
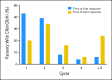
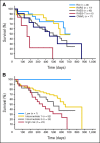
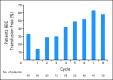
Results: Ninety-nine patients were enrolled; the ORR was 32% (17 complete responses [CR] plus 15 marrow CRs [mCRs]), and the overall improvement rate was 51%, which included 18% HI. Similar response rates were observed in all FAB subtypes and IPSS risk categories. Among patients who improved, 82% demonstrated responses by the end of cycle 2. Among 33 patients assessable for a cytogenetic response, 17 (52%) experienced cytogenetic CR (n = 11) or partial response (n = 6).
Conclusion: Decitabine given on a 5-day schedule provided meaningful clinical benefit for patients with MDS, with more than half demonstrating improvement. This suggests that decitabine can be administered in an outpatient setting with comparable efficacy and safety to the United States Food and Drug Administration-approved inpatient regimen.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Myelodysplastic Syndromes, V. 4:2006. National Comprehensive Cancer Network. 2006. http://www.nccn.org. - PMC - PubMed
-
- Santini V, Kantarjian HM, Issa JP. Changes in DNA methylation in neoplasia: Pathophysiology and therapeutic implications. Ann Intern Med. 2001;134:573–586. - PubMed
-
- Leone G, Teofili L, Voso MT, et al. DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematologica. 2002;87:1324–1341. - PubMed
-
- Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. - PubMed
-
- Pinto A, Zagonel V. 5-Aza-2′-deoxycytidine (Decitabine) and 5-azacytidine in the treatment of acute myeloid leukemias and myelodysplastic syndromes: Past, present and future trends. Leukemia. 1993;7(suppl 1):51–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

