Cloning of a high-affinity K+ transporter gene PutHKT2;1 from Puccinellia tenuiflora and its functional comparison with OsHKT2;1 from rice in yeast and Arabidopsis
- PMID: 19528529
- PMCID: PMC2724696
- DOI: 10.1093/jxb/erp184
Cloning of a high-affinity K+ transporter gene PutHKT2;1 from Puccinellia tenuiflora and its functional comparison with OsHKT2;1 from rice in yeast and Arabidopsis
Abstract
A high-affinity K+ transporter PutHKT2;1 cDNA was isolated from the salt-tolerant plant Puccinellia tenuiflora. Expression of PutHKT2;1 was induced by both 300 mM NaCl and K+-starvation stress in roots, but only slightly regulated by those stresses in shoots. PutHKT2;1 transcript levels in 300 mM NaCl were doubled by the depletion of potassium. Yeast transformed with PutHKT2;1, like those transformed with PhaHKT2;1 from salt-tolerant reed plants (Phragmites australis), (i) were able to take up K+ in low K+ concentration medium or in the presence of NaCl, and (ii) were permeable to Na+. This suggests that PutHKT2;1 has a high affinity K+-Na+ symport function in yeast. Arabidopsis over-expressing PutHKT2;1 showed increased sensitivities to Na+, K+, and Li+, while Arabidopsis over-expressing OsHKT2;1 from rice (Oryza sativa) showed increased sensitivity only to Na+. In contrast to OsHKT2;1, which functions in Na+-uptake at low external K+ concentrations, PutHKT2;1 functions in Na+-uptake at higher external K+ concentrations. These results show that the modes of action of PutHKT2;1 in transgenic yeast and Arabidopsis differ from the mode of action of the closely related OsHKT2;1 transporter.
Figures

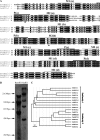








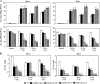
References
-
- Amtmann A, Sanders D. Mechanisms of Na+ uptake by plant cells. Advances in Botanical Research. 1999;29:75–112.
-
- Blumwald E, Aharon GS, Apse MP. Sodium transport in plant cells. Biochimica et Biophysica Acta. 2000;1465:140–151. - PubMed
-
- Cheong YH, Pandey GK, Grant JJ, Batistic O, Li L, Kim BG, lee SC, Kudla J, Luan S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. The Plant Journal. 2007;52:223–239. - PubMed
-
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. - PubMed

