Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior
- PMID: 19528658
- PMCID: PMC2705603
- DOI: 10.1073/pnas.0901800106
Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior
Abstract
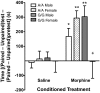
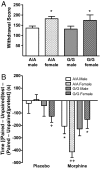
A single nucleotide polymorphism (SNP) in the human mu-opioid receptor gene (OPRM1 A118G) has been widely studied for its association in a variety of drug addiction and pain sensitivity phenotypes; however, the extent of these adaptations and the mechanisms underlying these associations remain elusive. To clarify the functional mechanisms linking the OPRM1 A118G SNP to addiction and analgesia phenotypes, we derived a mouse model possessing the equivalent nucleotide/amino acid substitution in the Oprm1 gene. Mice harboring this SNP (A112G) demonstrated several phenotypic similarities to humans carrying the A118G SNP, including reduced mRNA expression and morphine-mediated antinociception. We found additional phenotypes associated with this SNP including significant reductions of receptor protein levels, morphine-mediated hyperactivity, and the development of locomotor sensitization in mice harboring the G112 allele. In addition, we found sex-specific reductions in the rewarding properties of morphine and the aversive components of naloxone-precipitated morphine withdrawal. Further cross-species analysis will allow us to investigate mechanisms and adaptations present in humans carrying this SNP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


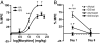
References
-
- Gelernter J, Kranzler H, Cubells J. Genetics of two mu opioid receptor gene (OPRM1) exon I polymorphisms: Population studies and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4(5):476–483. - PubMed
-
- Bergen AW, et al. Mu opioid receptor gene variants: Lack of association with alcohol dependence. Mol Psychiatry. 1997;2(6):490–494. - PubMed
-
- Tan EC, Tan CH, Karupathivan U, Yap EP. Mu opioid receptor gene polymorphisms and heroin dependence in Asian populations. Neuroreport. 2003;14(4):569–572. - PubMed
-
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28(12):1789–1795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

