Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose
- PMID: 19529771
- PMCID: PMC2691953
- DOI: 10.1371/journal.pone.0005930
Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose
Abstract
Background: Previously we have shown that Ag85B-TB10.4 is a highly efficient vaccine against tuberculosis when delivered in a Th1 inducing adjuvant based on cationic liposomes. Another Th1 inducing adjuvant, which has shown a very promising profile in both preclinical and clinical trials, is IC31. In this study, we examined the potential of Ag85B-TB10.4 delivered in the adjuvant IC31 for the ability to induce protection against infection with Mycobacterium tuberculosis. In addition, we examined if the antigen dose could influence the phenotype of the induced T cells.
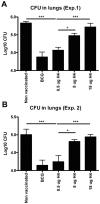
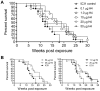
Methods and findings: We found that vaccination with the combination of Ag85B-TB10.4 and IC31 resulted in high numbers of polyfunctional CD4 T cells co-expressing IL-2, IFN-gamma and TNF-alpha. This correlated with protection against subsequent challenge with M.tb in the mouse TB model. Importantly, our results also showed that both the vaccine induced T cell response, and the protective efficacy, was highly dependent on the antigen dose. Thus, whereas antigen doses of 5 and 15 microg did not induce significant protection against M.tb, reducing the dose to 0.5 microg selectively increased the number of polyfunctional T cells and induced a strong protection against infection with M.tb. The influence of antigen dose was also observed in the guinea pig model of aerosol infection with M.tb. In this model a 2.5 fold increase in the antigen dose reduced the protection against infection with M.tb to the level observed in non-vaccinated animals.
Conclusions/significance: Small changes in the antigen dose can greatly influence the induction of specific T cell subpopulations and the dose is therefore a crucial factor when testing new vaccines. However, the adjuvant IC31 can, with the optimal dose of Ag85B-TB10.4, induce strong protection against Mycobacterium tuberculosis. This vaccine has now entered clinical trials.
Conflict of interest statement
Figures


References
-
- Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A. 2000;97:13853–13858. - PMC - PubMed
-
- McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–1244. - PubMed
-
- Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172:7618–7628. - PubMed
-
- Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, et al. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006;177:6353–6360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

