In situ aromatase expression in primary tumor is associated with estrogen receptor expression but is not predictive of response to endocrine therapy in advanced breast cancer
- PMID: 19531212
- PMCID: PMC2702392
- DOI: 10.1186/1471-2407-9-185
In situ aromatase expression in primary tumor is associated with estrogen receptor expression but is not predictive of response to endocrine therapy in advanced breast cancer
Abstract
Background: New, third-generation aromatase inhibitors (AIs) have proven comparable or superior to the anti-estrogen tamoxifen for treatment of estrogen receptor (ER) and/or progesterone receptor (PR) positive breast cancer. AIs suppress total body and intratumoral estrogen levels. It is unclear whether in situ carcinoma cell aromatization is the primary source of estrogen production for tumor growth and whether the aromatase expression is predictive of response to endocrine therapy. Due to methodological difficulties in the determination of the aromatase protein, COX-2, an enzyme involved in the synthesis of aromatase, has been suggested as a surrogate marker for aromatase expression.
Methods: Primary tumor material was retrospectively collected from 88 patients who participated in a randomized clinical trial comparing the AI letrozole to the anti-estrogen tamoxifen for first-line treatment of advanced breast cancer. Semi-quantitative immunohistochemical (IHC) analysis was performed for ER, PR, COX-2 and aromatase using Tissue Microarrays (TMAs). Aromatase was also analyzed using whole sections (WS). Kappa analysis was applied to compare association of protein expression levels. Univariate Wilcoxon analysis and the Cox-analysis were performed to evaluate time to progression (TTP) in relation to marker expression.
Results: Aromatase expression was associated with ER, but not with PR or COX-2 expression in carcinoma cells. Measurements of aromatase in WS were not comparable to results from TMAs. Expression of COX-2 and aromatase did not predict response to endocrine therapy. Aromatase in combination with high PR expression may select letrozole treated patients with a longer TTP.
Conclusion: TMAs are not suitable for IHC analysis of in situ aromatase expression and we did not find COX-2 expression in carcinoma cells to be a surrogate marker for aromatase. In situ aromatase expression in tumor cells is associated with ER expression and may thus point towards good prognosis. Aromatase expression in cancer cells is not predictive of response to endocrine therapy, indicating that in situ estrogen synthesis may not be the major source of intratumoral estrogen. However, aromatase expression in combination with high PR expression may select letrozole treated patients with longer TTP.
Trial registration: Sub-study of trial P025 for advanced breast cancer.
Figures

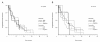
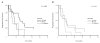
References
-
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. - PubMed
-
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–2109. doi: 10.1200/JCO.2003.04.194. - DOI - PubMed
-
- Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18:3758–3767. - PubMed
-
- Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T. Phase III Study Comparing Exemestane With Tamoxifen As First-Line Hormonal Treatment of Metastatic Breast Cancer in Postmenopausal Women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–4890. doi: 10.1200/JCO.2007.14.4659. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

