Distinct origins and genetic programs of head muscle satellite cells
- PMID: 19531353
- PMCID: PMC3684422
- DOI: 10.1016/j.devcel.2009.05.007
Distinct origins and genetic programs of head muscle satellite cells
Abstract
Adult skeletal muscle possesses a remarkable regenerative capacity, due to the presence of satellite cells, adult muscle stem cells. We used fate-mapping techniques in avian and mouse models to show that trunk (Pax3(+)) and cranial (MesP1(+)) skeletal muscle and satellite cells derive from separate genetic lineages. Similar lineage heterogeneity is seen within the head musculature and satellite cells, due to their shared, heterogenic embryonic origins. Lineage tracing experiments with Isl1Cre mice demonstrated the robust contribution of Isl1(+) cells to distinct jaw muscle-derived satellite cells. Transplantation of myofiber-associated, Isl1-derived satellite cells into damaged limb muscle contributed to muscle regeneration. In vitro experiments demonstrated the cardiogenic nature of cranial- but not trunk-derived satellite cells. Finally, overexpression of Isl1 in the branchiomeric muscles of chick embryos inhibited skeletal muscle differentiation in vitro and in vivo, suggesting that this gene plays a role in the specification of cardiovascular and skeletal muscle stem cell progenitors.
Figures


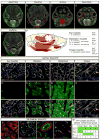

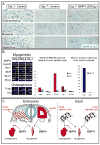

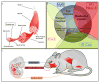
Comment in
-
Head muscles: aliens who came in from the cold?Dev Cell. 2009 Jun;16(6):779-80. doi: 10.1016/j.devcel.2009.06.004. Dev Cell. 2009. PMID: 19531348
References
-
- Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. - PubMed
-
- Armand O, Boutineau AM, Mauger A, Pautou MP, Kieny M. Origin of satellite cells in avian skeletal muscles. Arch Anat Microsc Morphol Exp. 1983;72:163–181. - PubMed
-
- Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. - PubMed
-
- Bothe I, Ahmed MU, Winterbottom FL, von Scheven G, Dietrich S. Extrinsic versus intrinsic cues in avian paraxial mesoderm patterning and differentiation. Dev Dyn. 2007;236:2397–2409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

