Effects of age on meiosis in budding yeast
- PMID: 19531355
- PMCID: PMC2948205
- DOI: 10.1016/j.devcel.2009.05.013
Effects of age on meiosis in budding yeast
Abstract
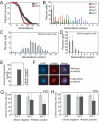
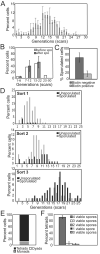
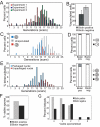
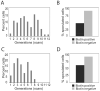
In humans, the frequency with which meiotic chromosome mis-segregation occurs increases with age. Whether age-dependent meiotic defects occur in other organisms is unknown. Here, we examine the effects of replicative aging on meiosis in budding yeast. We find that aged mother cells show a decreased ability to initiate the meiotic program and fail to express the meiotic inducer IME1. The few aged mother cells that do enter meiosis complete this developmental program but exhibit defects in meiotic chromosome segregation and spore formation. Furthermore, we find that mutations that extend replicative life span also extend the sexual reproductive life span. Our results indicate that in budding yeast, the ability to initiate and complete the meiotic program as well as the fidelity of meiotic chromosome segregation decrease with cellular age and are controlled by the same pathways that govern aging of asexually reproducing yeast cells.
Figures



References
-
- Covitz PA, Herskowitz I, Mitchell AP. The yeast RME1 gene encodes a putative zinc finger protein that is directly repressed by a1-alpha 2. Genes Dev. 1991;5:1982–9. - PubMed
-
- Chen C, Dewaele S, Braeckman B, Desmyter L, Verstraelen J, Borgonieb G, Vanfleterenb J, Contrerasa R. A high-throughput screening system for genes extending life-span. Experimental Gerontology. 2003;38:1051–1063. - PubMed
-
- Defossez PA, Prusty R, Kaeberlein M, Lin SJ, Ferrigno P, Silver PA, Keil RL, Guarente L. Elimination of the replication block protein Fob1 extends the life span of yeast mother cells. Mol. Cell. 1999;3:447–55. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

