Zebrafish Hagoromo mutants up-regulate fgf8 postembryonically and develop neuroblastoma
- PMID: 19531571
- PMCID: PMC2744123
- DOI: 10.1158/1541-7786.MCR-08-0555
Zebrafish Hagoromo mutants up-regulate fgf8 postembryonically and develop neuroblastoma
Abstract
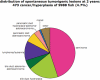
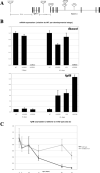
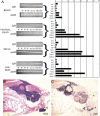
We screened an existing collection of zebrafish insertional mutants for cancer susceptibility by histologic examination of heterozygotes at 2 years of age. As most mutants had no altered cancer predisposition, this provided the first comprehensive description of spontaneous tumor spectrum and frequency in adult zebrafish. Moreover, the screen identified four lines, each carrying a different dominant mutant allele of Hagoromo previously linked to adult pigmentation defects, which develop tumors with high penetrance and that histologically resemble neuroblastoma. These tumors are clearly neural in origin, although they do not express catecholaminergic neuronal markers characteristic of human neuroblastoma. The zebrafish tumors result from inappropriate maintenance of a cell population within the cranial ganglia that are likely neural precursors. These neoplasias typically remain small but they can become highly aggressive, initially traveling along cranial nerves, and ultimately filling the head. The developmental origin of these tumors is highly reminiscent of human neuroblastoma. The four mutant Hagoromo alleles all contain viral insertions in the fbxw4 gene, which encodes an F-box WD40 domain-containing protein. However, although one allele clearly reduced the levels of fbxw4 mRNA, the other three insertions had no detectable effect on fbw4 expression. Instead, we showed that all four mutations result in the postembryonic up-regulation of the neighboring gene, fibroblast growth factor 8 (fgf8). Moreover, fgf8 is highly expressed in the tumorigenic lesions. Although fgf8 overexpression is known to be associated with breast and prostate cancer in mammals, this study provides the first evidence that fgf8 misregulation can lead to neural tumors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

