Identification of protein cofactors necessary for sequence-specific plasmid DNA nuclear import
- PMID: 19532138
- PMCID: PMC2835029
- DOI: 10.1038/mt.2009.127
Identification of protein cofactors necessary for sequence-specific plasmid DNA nuclear import
Abstract
Although transfections are routinely used in the laboratory, the mechanism(s) by which exogenous DNA is transported into the nucleus is poorly understood. By improving our understanding of how vectors circumvent the numerous cellular barriers to gene transfer, more efficient gene delivery methods can be devised. We have begun to design plasmid constructs that enter the nucleus of specific cell types in the absence of cell division, thereby enhancing levels of expression. We have shown that inclusion of specific DNA sequences in plasmid constructs mediates nuclear import both in vitro and in vivo. Here, we use plasmid affinity chromatography, mass spectrometry (MS), and live-cell pulldowns of transfected plasmid constructs to identify protein cofactors that interact in a sequence-specific manner with these DNA nuclear targeting sequences (DTSs). Importin beta(1), importin 7, and the small guanosine triphosphatase Ran all demonstrate DTS-specific interaction in both MS and pull-down assays, consistent with our model of plasmid nuclear import. In addition, knockdown of importin beta(1) with small interfering RNA (siRNA) abrogates plasmid nuclear import, indicating that it is a necessary cofactor. Our discovery that specific karyopherins mediate plasmid nuclear import can be used to design more effective vectors for gene delivery.
Figures
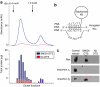
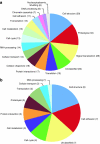

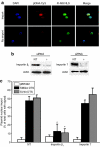
References
-
- Brunner S, Sauer T, Carotta S, Cotten M, Saltik M., and , Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000;7:401–407. - PubMed
-
- Zabner J, Fasbender AJ, Moninger T, Poellinger KA., and , Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. - PubMed
-
- Liu F, Song Y., and , Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. - PubMed
-
- Barron LG, Szoka FC.1999The perplexing delivery mechanism of lipoplexesIn: Huang, L, Hung, M-C, Wagner, E (eds). Nonviral Vectors for Gene Therapy Academic: San Diego; 230–266.
-
- Godbey WT, Barry MA, Saggau P, Wu KK., and , Mikos AG. Poly(ethylenimine)-mediated transfection: a new paradigm for gene delivery. J Biomed Mater Res. 2000;51:321–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

