Microfluidics for cryopreservation
- PMID: 19532962
- PMCID: PMC2719835
- DOI: 10.1039/b823062e
Microfluidics for cryopreservation
Abstract
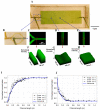
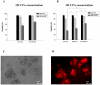
Minimizing cell damage throughout the cryopreservation process is critical to enhance the overall outcome. Osmotic shock sustained during the loading and unloading of cryoprotectants (CPAs) is a major source of cell damage during the cryopreservation process. We introduce a microfluidic approach to minimize osmotic shock to cells during cryopreservation. This approach allows us to control the loading and unloading of CPAs in microfluidic channels using diffusion and laminar flow. We provide a theoretical explanation of how the microfluidic approach minimizes osmotic shock in comparison to conventional cryopreservation protocols via cell membrane transport modeling. Finally, we show that biological experiments are consistent with the proposed mathematical model. The results indicate that our novel microfluidic-based approach improves post-thaw cell survivability by up to 25% on average over conventional cryopreservation protocols. The method developed in this study provides a platform to cryopreserve cells with higher viability, functionality, and minimal inter-technician variability. This method introduces microfluidic technologies to the field of biopreservation, opening the door to future advancements at the interface of these fields.
Figures

References
-
- Burns MA, Johnson BN, Brahmasandra SN, Handique K, Webster JR, Krishnan M, Sammarco TS, Man PM, Jones D, Heldsinger D, Mastrangelo CH, Burke DT. Science. 1998;282:484–487. - PubMed
-
- Hong JW, Quake SR. Nat Biotechnol. 2003;21:1179–1183. - PubMed
-
- Okamoto T, Suzuki T, Yamamoto N. Nat Biotechnol. 2000;18:438–441. - PubMed
-
- Khademhosseini A, Yeh J, Eng G, Karp J, Kaji H, Borenstein J, Farokhzad OC, Langer R. Lab Chip. 2005;5:1380–1386. - PubMed
-
- Demirci U. Journal Of Microelectromechanical Systems. 2006;15:957–966.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

