The P2X(7) receptor mediates the uptake of organic cations in canine erythrocytes and mononuclear leukocytes: comparison to equivalent human cell types
- PMID: 19533417
- PMCID: PMC2717320
- DOI: 10.1007/s11302-009-9163-1
The P2X(7) receptor mediates the uptake of organic cations in canine erythrocytes and mononuclear leukocytes: comparison to equivalent human cell types
Abstract
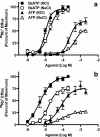
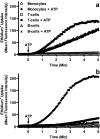
We previously demonstrated that canine erythrocytes express the P2X(7) receptor, and that the function and expression of this receptor is greatly increased compared with human erythrocytes. Using (86)Rb(+) (K(+)) and organic cation flux measurements, we further compared P2X(7) in erythrocytes and mononuclear leukocytes from these species. Concentration response curves of BzATP- and ATP-induced (86)Rb(+) efflux demonstrated that canine P2X(7) was less sensitive to inhibition by extracellular Na(+) ions compared to human P2X(7). In contrast, canine and human P2X(7) showed a similar sensitivity to the P2X(7) antagonists KN-62 and Mg(2+). KN-62 and Mg(2+) also inhibited ATP-induced choline(+) uptake into canine and human erythrocytes. BzATP and ATP but not ADP or NAD induced ethidium(+) uptake into canine monocytes, T- and B-cells. ATP-induced ethidium(+) uptake was twofold greater in canine T-cells compared to canine B-cells and monocytes. KN-62 inhibited the ATP-induced ethidium(+) uptake in each cell type. P2X(7)-mediated uptake of organic cations was 40- and fivefold greater in canine erythrocytes and lymphocytes (T- and B-cells), respectively, compared to equivalent human cell types. In contrast, P2X(7) function was threefold lower in canine monocytes compared to human monocytes. Thus, P2X(7) activation can induce the uptake of organic cations into canine erythrocytes and mononuclear leukocytes, but the relative levels of P2X(7) function differ to that of equivalent human cell types.
Figures




References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.bbrc.2008.10.042', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.bbrc.2008.10.042'}, {'type': 'PubMed', 'value': '18938136', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18938136/'}]}
- Nicke A (2008) Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun 377:803–808. doi:10.1016/j.bbrc.2008.10.042 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s00210-007-0222-2', 'is_inner': False, 'url': 'https://doi.org/10.1007/s00210-007-0222-2'}, {'type': 'PMC', 'value': 'PMC7100647', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC7100647/'}, {'type': 'PubMed', 'value': '18273661', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18273661/'}]}
- Köles L, Gerevich Z, Oliveira JF, Zadori ZS, Wirkner K, Illes P (2008) Interaction of P2 purinergic receptors with cellular macromolecules. Naunyn Schmiedebergs Arch Pharmacol 377:1–33. doi:10.1007/s00210-007-0222-2 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC2686822', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2686822/'}, {'type': 'PubMed', 'value': '19189228', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/19189228/'}]}
- Qu Y, Dubyak GR (2009) P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. doi:10.1007/s11302-009-9132-8 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s00424-006-0070-9', 'is_inner': False, 'url': 'https://doi.org/10.1007/s00424-006-0070-9'}, {'type': 'PubMed', 'value': '16649055', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16649055/'}]}
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537. doi:10.1007/s00424-006-0070-9 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.tips.2007.07.002', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.tips.2007.07.002'}, {'type': 'PubMed', 'value': '17692395', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17692395/'}]}
- Di Virgilio F (2007) Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci 28:465–472. doi:10.1016/j.tips.2007.07.002 - PubMed
LinkOut - more resources
Full Text Sources

