Viral calciomics: interplays between Ca2+ and virus
- PMID: 19535138
- PMCID: PMC3449087
- DOI: 10.1016/j.ceca.2009.05.005
Viral calciomics: interplays between Ca2+ and virus
Abstract
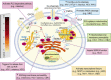
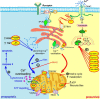
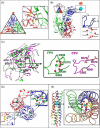
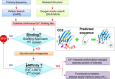
Ca(2+) is one of the most universal and versatile signaling molecules and is involved in almost every aspect of cellular processes. Viruses are adept at utilizing the universal Ca(2+) signal to create a tailored cellular environment that meets their own demands. This review summarizes most of the known mechanisms by which viruses perturb Ca(2+) homeostasis and utilize Ca(2+) and cellular Ca(2+)-binding proteins to their benefit in their replication cycles. Ca(2+) plays important roles in virion structure formation, virus entry, viral gene expression, posttranslational processing of viral proteins and virion maturation and release. As part of the review, we introduce an algorithm to identify linear "EF-hand" Ca(2+)-binding motifs which resulted in the prediction of a total of 93 previously unrecognized Ca(2+)-binding motifs in virus proteins. Many of these proteins are nonstructural proteins, a class of proteins among which Ca(2+) interactions had not been formerly appreciated. The presence of linear Ca(2+)-binding motifs in viral proteins enlarges the spectrum of Ca(2+)-virus interplay and expands the total scenario of viral calciomics.
Figures

References
-
- Berridge M.J., Bootman M.D., Lipp P. Calcium—a life and death signal. Nature. 1998;395:645–648. - PubMed
-
- Verkhratsky A. Calcium and cell death. Subcell. Biochem. 2007;45:465–480. - PubMed
-
- Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 2003;4:517–529. - PubMed
-
- Mikoshiba K., Hattori M. IP3 receptor-operated calcium entry. Sci. STKE. 2000;2000:PE1. - PubMed
-
- Zeng W., Mak D.O., Li Q., Shin D.M., Foskett J.K., Muallem S. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gbetagamma. Curr. Biol. 2003;13:872–876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

