Rastering strategy for screening and centring of microcrystal samples of human membrane proteins with a sub-10 microm size X-ray synchrotron beam
- PMID: 19535414
- PMCID: PMC2843980
- DOI: 10.1098/rsif.2009.0142.focus
Rastering strategy for screening and centring of microcrystal samples of human membrane proteins with a sub-10 microm size X-ray synchrotron beam
Abstract
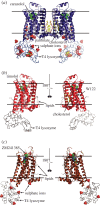
Crystallization of human membrane proteins in lipidic cubic phase often results in very small but highly ordered crystals. Advent of the sub-10 microm minibeam at the APS GM/CA CAT has enabled the collection of high quality diffraction data from such microcrystals. Herein we describe the challenges and solutions related to growing, manipulating and collecting data from optically invisible microcrystals embedded in an opaque frozen in meso material. Of critical importance is the use of the intense and small synchrotron beam to raster through and locate the crystal sample in an efficient and reliable manner. The resulting diffraction patterns have a significant reduction in background, with strong intensity and improvement in diffraction resolution compared with larger beam sizes. Three high-resolution structures of human G protein-coupled receptors serve as evidence of the utility of these techniques that will likely be useful for future structural determination efforts. We anticipate that further innovations of the technologies applied to microcrystallography will enable the solving of structures of ever more challenging targets.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

