Matrix mediates the functional link between human immunodeficiency virus type 1 RNA nuclear export elements and the assembly competency of Gag in murine cells
- PMID: 19535446
- PMCID: PMC2738188
- DOI: 10.1128/JVI.00699-09
Matrix mediates the functional link between human immunodeficiency virus type 1 RNA nuclear export elements and the assembly competency of Gag in murine cells
Abstract
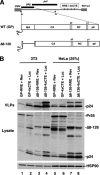
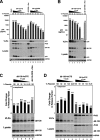
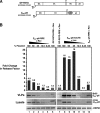
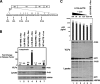
Human immunodeficiency virus type 1 (HIV-1) assembles poorly in murine cells, reflecting inefficient targeting of the Gag structural polyprotein to the plasma membrane. Virus particle production can be restored by replacing the cis-acting Rev response element (RRE) in Gag-Pol mRNAs with multiple copies of the CTE (4xCTE), suggesting a mechanistic link between HIV-1 RNA trafficking and productive Gag assembly. In this report, we demonstrate that Gag molecules generated from RRE-dependent transcripts are intrinsically defective for assembly in murine 3T3 cells. When controlled for the intracellular Gag level, modulations of the Gag matrix (MA) domain that enhance Gag membrane association (e.g., deletion of the MA globular head) substantially improve assembly for Gag derived from RRE- but not 4xCTE-dependent transcripts. Gag mutants carrying a leucine zipper replacement of the nucleocapsid (NC) domain remain largely assembly defective when derived from RRE-dependent transcripts, indicating that the defect does not reflect aberrant NC/RNA-driven Gag multimerization. We further demonstrate that single changes in uncharged amino acids implicated in Gag/MA myristoyl switch regulation, most notably replacing the leucine at position 21 with serine, improve assembly for Gag derived from RRE-dependent transcripts. In sum, we provide genetic evidence to suggest that HIV-1 RNA metabolism specifically modulates the activation of MA-dependent membrane targeting.
Figures

References
-
- Atchison, R. E., J. Gosling, F. S. Monteclaro, C. Franci, L. Digilio, I. F. Charo, and M. A. Goldsmith. 1996. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science 2741924-1926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

