Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI-1-mediated inhibition
- PMID: 19535514
- PMCID: PMC2724094
- DOI: 10.1152/ajpcell.00201.2009
Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI-1-mediated inhibition
Abstract
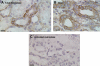
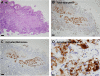
Matriptase, a transmembrane serine protease, is broadly expressed by, and crucial for the integrity of, the epithelium. Matriptase is synthesized as a zymogen and undergoes autoactivation to become an active protease that is immediately inhibited by, and forms complexes with, hepatocyte growth factor activator inhibitor (HAI-1). To investigate where matriptase is activated and how it is secreted in vivo, we determined the expression and activation status of matriptase in seminal fluid and urine and the distribution and subcellular localization of the protease in the prostate and kidney. The in vivo studies revealed that while the latent matriptase is localized at the basolateral surface of the ductal epithelial cells of both organs, only matriptase-HAI-1 complexes and not latent matriptase are detected in the body fluids, suggesting that activation, inhibition, and transcytosis of matriptase would have to occur for the secretion of matriptase. These complicated processes involved in the in vivo secretion were also observed in polarized Caco-2 intestinal epithelial cells. The cells target latent matriptase to the basolateral plasma membrane where activation, inhibition, and secretion of matriptase appear to take place. However, a proportion of matriptase-HAI-1 complexes, but not the latent matriptase, appears to undergo transcytosis to the apical plasma membrane for secretion. When epithelial cells lose their polarity, they secrete both latent and activated matriptase. Although most epithelial cells retain very low levels of matriptase-HAI-1 complex by rapidly secreting the complex, gastric chief cells may activate matriptase and store matriptase-HAI-1 complexes in the pepsinogen-secretory granules, suggesting an intracellular activation and regulated secretion in these cells. Taken together, while zymogen activation and closely coupled HAI-1-mediated inhibition are common features for matriptase regulation, the cellular location of matriptase activation and inhibition, and the secretory route for matriptase-HAI-1 complex may vary along with the functional divergence of different epithelial cells.
Figures
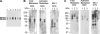



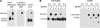

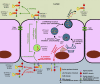
Similar articles
-
Differential subcellular localization renders HAI-2 a matriptase inhibitor in breast cancer cells but not in mammary epithelial cells.PLoS One. 2015 Mar 18;10(3):e0120489. doi: 10.1371/journal.pone.0120489. eCollection 2015. PLoS One. 2015. PMID: 25786220 Free PMC article.
-
The protease inhibitor HAI-2, but not HAI-1, regulates matriptase activation and shedding through prostasin.J Biol Chem. 2014 Aug 8;289(32):22319-32. doi: 10.1074/jbc.M114.574400. Epub 2014 Jun 24. J Biol Chem. 2014. PMID: 24962579 Free PMC article.
-
Simultaneous activation and hepatocyte growth factor activator inhibitor 1-mediated inhibition of matriptase induced at activation foci in human mammary epithelial cells.Am J Physiol Cell Physiol. 2005 Apr;288(4):C932-41. doi: 10.1152/ajpcell.00497.2004. Epub 2004 Dec 8. Am J Physiol Cell Physiol. 2005. PMID: 15590895
-
Role of the polycystic kidney disease domain in matriptase chaperone activity and localization of hepatocyte growth factor activator inhibitor-1.FEBS J. 2022 Jun;289(12):3422-3439. doi: 10.1111/febs.16348. Epub 2022 Jan 23. FEBS J. 2022. PMID: 35020274 Review.
-
Matriptase: potent proteolysis on the cell surface.Mol Med. 2006 Jan-Mar;12(1-3):1-7. doi: 10.2119/2006-00022.List. Mol Med. 2006. PMID: 16838070 Free PMC article. Review.
Cited by
-
Transport via the transcytotic pathway makes prostasin available as a substrate for matriptase.J Biol Chem. 2011 Feb 18;286(7):5793-802. doi: 10.1074/jbc.M110.186874. Epub 2010 Dec 10. J Biol Chem. 2011. PMID: 21148558 Free PMC article.
-
Matriptase expression and zymogen activation in human pilosebaceous unit.J Histochem Cytochem. 2014 Jan;62(1):50-9. doi: 10.1369/0022155413505599. Epub 2013 Sep 4. J Histochem Cytochem. 2014. PMID: 24004857 Free PMC article.
-
Roles of CUB and LDL receptor class A domain repeats of a transmembrane serine protease matriptase in its zymogen activation.J Biochem. 2013 Jan;153(1):51-61. doi: 10.1093/jb/mvs118. Epub 2012 Oct 3. J Biochem. 2013. PMID: 23038671 Free PMC article.
-
Intestinal regulation of suppression of tumorigenicity 14 (ST14) and serine peptidase inhibitor, Kunitz type -1 (SPINT1) by transcription factor CDX2.Sci Rep. 2018 Aug 7;8(1):11813. doi: 10.1038/s41598-018-30216-z. Sci Rep. 2018. PMID: 30087389 Free PMC article.
-
Matriptase shedding is closely coupled with matriptase zymogen activation and requires de novo proteolytic cleavage likely involving its own activity.PLoS One. 2017 Aug 22;12(8):e0183507. doi: 10.1371/journal.pone.0183507. eCollection 2017. PLoS One. 2017. PMID: 28829816 Free PMC article.
References
-
- Ahmed S, Jin X, Yagi M, Yasuda C, Sato Y, Higashi S, Lin CY, Dickson RB, Miyazaki K. Identification of membrane-bound serine proteinase matriptase as processing enzyme of insulin-like growth factor binding protein-related protein-1 (IGFBP-rP1/angiomodulin/mac25). FEBS J 273: 615–627, 2006. - PubMed
-
- Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem 268: 1439–1447, 2001. - PubMed
-
- Benaud C, Oberst M, Hobson JP, Spiegel S, Dickson RB, Lin CY. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J Biol Chem 277: 10539–10546, 2002. - PubMed
-
- Benaud CM, Oberst M, Dickson RB, Lin CY. Deregulated activation of matriptase in breast cancer cells. Clin Exp Metastasis 19: 639–649, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

