Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons
- PMID: 19535786
- PMCID: PMC2754884
- DOI: 10.1095/biolreprod.109.078261
Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons
Abstract
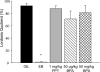
Developmental exposure to endocrine-disrupting compounds is hypothesized to adversely affect female reproductive physiology by interfering with the organization of the hypothalamic-pituitary-gonadal axis. Here, we compared the effects of neonatal exposure to two environmentally relevant doses of the plastics component bisphenol-A (BPA; 50 microg/kg and 50 mg/kg) with the ESR1 (formerly known as ERalpha)-selective agonist 4,4',4''-(4-propyl-[(1)H]pyrazole-1,3,5-triyl)trisphenol (PPT; 1 mg/kg) on the development of the female rat hypothalamus and ovary. An oil vehicle and estradiol benzoate (EB; 25 microg) were used as negative and positive controls. Exposure to EB, PPT, or the low dose of BPA advanced pubertal onset. A total of 67% of females exposed to the high BPA dose were acyclic by 15 wk after vaginal opening compared with 14% of those exposed to the low BPA dose, all of the EB- and PPT-treated females, and none of the control animals. Ovaries from the EB-treated females were undersized and showed no evidence of folliculogenesis, whereas ovaries from the PPT-treated females were characterized by large antral-like follicles, which did not appear to support ovulation. Severity of deficits within the BPA-treated groups increased with dose and included large antral-like follicles and lower numbers of corpora lutea. Sexual receptivity, examined after ovariectomy and hormone replacement, was normal in all groups except those neonatally exposed to EB. FOS induction in hypothalamic gonadotropic (GnRH) neurons after hormone priming was impaired in the EB- and PPT-treated groups but neither of the BPA-treated groups. Our data suggest that BPA disrupts ovarian development but not the ability of GnRH neurons to respond to steroid-positive feedback.
Figures




References
-
- Dodds EC, Lawson W.Synthetic estrogen agents without the phenanthrene nucleus. Nature 1936; 137: 996
-
- Tsutsumi O.Assessment of human contamination of estrogenic endocrine-disrupting chemicals and their risk for human reproduction. J Steroid Biochem Mol Biol 2005; 93: 325–330.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

