TAK1 is an essential regulator of BMP signalling in cartilage
- PMID: 19536134
- PMCID: PMC2699391
- DOI: 10.1038/emboj.2009.162
TAK1 is an essential regulator of BMP signalling in cartilage
Abstract
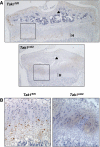
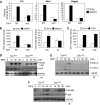
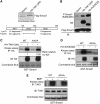
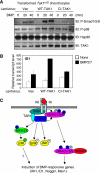
TGFbeta activated kinase 1 (TAK1), a member of the MAPKKK family, controls diverse functions ranging from innate and adaptive immune system activation to vascular development and apoptosis. To analyse the in vivo function of TAK1 in cartilage, we generated mice with a conditional deletion of Tak1 driven by the collagen 2 promoter. Tak1(col2) mice displayed severe chondrodysplasia with runting, impaired formation of secondary centres of ossification, and joint abnormalities including elbow dislocation and tarsal fusion. This phenotype resembled that of bone morphogenetic protein receptor (BMPR)1 and Gdf5-deficient mice. BMPR signalling was markedly impaired in TAK1-deficient chondrocytes as evidenced by reduced expression of known BMP target genes as well as reduced phosphorylation of Smad1/5/8 and p38/Jnk/Erk MAP kinases. TAK1 mediates Smad1 phosphorylation at C-terminal serine residues. These findings provide the first in vivo evidence in a mammalian system that TAK1 is required for BMP signalling and functions as an upstream activating kinase for Smad1/5/8 in addition to its known role in regulating MAP kinase pathways. Our experiments reveal an essential role for TAK1 in the morphogenesis, growth, and maintenance of cartilage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL (1997) TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem 272: 27678–27685 - PubMed
-
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R (2004) Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol 276: 124–142 - PubMed
-
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A (1999) Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 126: 1631–1642 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

