A newly discovered protein export machine in malaria parasites
- PMID: 19536257
- PMCID: PMC2725363
- DOI: 10.1038/nature08104
A newly discovered protein export machine in malaria parasites
Abstract
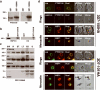
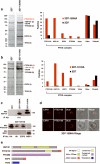
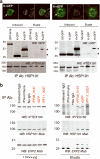
Several hundred malaria parasite proteins are exported beyond an encasing vacuole and into the cytosol of the host erythrocyte, a process that is central to the virulence and viability of the causative Plasmodium species. The trafficking machinery responsible for this export is unknown. Here we identify in Plasmodium falciparum a translocon of exported proteins (PTEX), which is located in the vacuole membrane. The PTEX complex is ATP-powered, and comprises heat shock protein 101 (HSP101; a ClpA/B-like ATPase from the AAA+ superfamily, of a type commonly associated with protein translocons), a novel protein termed PTEX150 and a known parasite protein, exported protein 2 (EXP2). EXP2 is the potential channel, as it is the membrane-associated component of the core PTEX complex. Two other proteins, a new protein PTEX88 and thioredoxin 2 (TRX2), were also identified as PTEX components. As a common portal for numerous crucial processes, this translocon offers a new avenue for therapeutic intervention.
Figures

Comment in
-
Malaria: The gatekeeper revealed.Nature. 2009 Jun 18;459(7249):918-9. doi: 10.1038/459918a. Nature. 2009. PMID: 19536248 No abstract available.
References
-
- Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. - PubMed
-
- Marti M, et al. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. - PubMed
-
- Hiller NL, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

