Clinical prognostic value of RNA viral load and CD4 cell counts during untreated HIV-1 infection--a quantitative review
- PMID: 19536329
- PMCID: PMC2694276
- DOI: 10.1371/journal.pone.0005950
Clinical prognostic value of RNA viral load and CD4 cell counts during untreated HIV-1 infection--a quantitative review
Abstract
Background: The prognostic value of CD4 counts and RNA viral load for identifying treatment need in HIV-infected individuals depends on (a) variation within and among individuals, and (b) relative risks of clinical progression per unit CD4 or RNA difference.
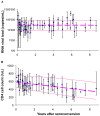
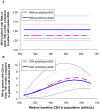
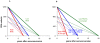
Methodology/principal findings: We reviewed these measurements across (a) 30 studies, and (b) 16 cohorts of untreated seropositive adults. Median within-population interquartile ranges were 74,000 copies/mL for RNA with no significant change during the course of infection; and 330 cells/microL for CD4, with a slight proportional increase over infection. Applying measurement and physiological fluctuations observed on chronically infected patients, we estimate that 45% of population-level variation in RNA, and 25% of variation in CD4, were due to within-patient fluctuations. Comparing a patient with RNA at upper 75(th) centile with a patient at median RNA, 5-year relative risks were 1.4 (95% CI 1.2-1.7) for AIDS and 1.5 (1.3-1.9) for death, without change over the course of infection. In contrast, for a patient with CD4 count at the lower 75(th) centile, relative risks increased from 1.0 at seroconversion to maxima of 6.3 (4.4-8.9) for AIDS and 5.5 (2.7-10.1) for death by year 6, when the population median had fallen to 300 cells/microL. Below 300 cells/microL, prognostic power did not increase, due to a narrower CD4 range.
Conclusions: Findings support the current WHO recommendation (used with clinical criteria) to start antiretroviral treatment in low-income settings at CD4 thresholds of 200-350 cells/microL, without pre-treatment RNA monitoring--while not precluding earlier treatment based on clinical, socio-demographic or public health criteria.
Conflict of interest statement
Figures


References
-
- USA department of Health and Human Services Panel on antiretroviral guidelines for adults and adolescents–a working group of the Office of AIDS Research Advisory Council. Guidelines for the use of antiretroviral agents in HIV-1-Infected adults and adolescents. 29 January, 2008. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
-
- Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society–USA panel. Top HIV Med. 2006;14:827–43. - PubMed
-
- Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. - PubMed
-
- de Wolf F, Spijkerman I, Schellekens PT, et al. AIDS prognosis based on HIV-1 RNA, CD4+ T-cell count and function: markers with reciprocal predictive value over time after seroconversion. Aids. 1997;11:1799–806. - PubMed
-
- Henrard DR, Phillips JF, Muenz LR, et al. Natural history of HIV-1 cell-free viremia. Jama. 1995;274:554–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

