Synthetic tumor-specific breakpoint peptide vaccine in patients with chronic myeloid leukemia and minimal residual disease: a phase 2 trial
- PMID: 19536894
- PMCID: PMC5534348
- DOI: 10.1002/cncr.24468
Synthetic tumor-specific breakpoint peptide vaccine in patients with chronic myeloid leukemia and minimal residual disease: a phase 2 trial
Abstract
Background: Imatinib is the current standard frontline therapy for chronic myelogenous leukemia (CML). In the majority of patients, imatinib induces a complete cytogenetic response (CCyR); however, complete molecular responses are infrequent. The Bcr-Abl fusion creates a unique sequence of amino acids that could constitute a target for immunomodulation.
Methods: A mixture of heteroclitic and native peptides derived from both b3a2 and b2a2 sequences was used to vaccinate patients with CML in CCyR who were receiving imatinib therapy and who had stable Bcr-Abl transcript levels.
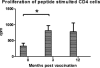
Results: Ten patients were enrolled, all with b2a2 transcripts (including 2 patients who had coexpression of b2a2 and b3a2). Patients had received imatinib for a median of 62 months. Three of 10 patients achieved 1-log reduction in Bcr-Abl transcript levels, including the 2 patients who had received previous interferon therapy, and 3 other patients achieved a major molecular response. The vaccine was tolerated well, and there were no grade > or =3 adverse events. Vaccination did not affect the leukocyte profiles in peripheral blood except for regulatory T cells, which were down-regulated briefly during the late stage of vaccination in patients who achieved approximately 1-log reduction in Bcr-Abl transcript levels.
Conclusions: The current data suggested that vaccination-related transient disruption of immune tolerance may contribute to the reduction in Bcr-Abl transcripts. Clinically, this Bcr-Abl peptide vaccine may transiently improve the molecular response in a subset of patients with CML.
Conflict of interest statement
Conflict of Interest Disclosures
Figures


References
-
- de Klein A, van Kessel AG, Grosveld G, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. - PubMed
-
- Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining [letter] Nature. 1973;243:290–293. - PubMed
-
- Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550–554. - PubMed
-
- Shtivelman E, Lifshitz B, Gale RP, Roe BA, Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986;47:277–284. - PubMed
-
- Shepherd P, Suffolk R, Halsey J, Allan N. Analysis of molecular breakpoint and m-RNA transcripts in a prospective randomized trial of interferon in chronic myeloid leukaemia: no correlation with clinical features, cytogenetic response, duration of chronic phase, or survival. Br J Haematol. 1995;89:546–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

