Bioresponsive, cell-penetrating, and multimeric MR contrast agents
- PMID: 19537782
- PMCID: PMC2739617
- DOI: 10.1021/ar800245h
Bioresponsive, cell-penetrating, and multimeric MR contrast agents
Abstract
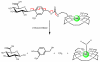
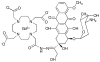
Magnetic resonance imaging (MRI) has become increasingly popular in experimental molecular imaging and clinical radiology because it is non-invasive and capable of producing three-dimensional representations of opaque organisms with high spatial and temporal resolution. Approximately 35% of all clinical MR scans utilize contrast media, however a primary limitation of MR imaging is the sensitivity of contrast agents that require high concentrations (0.1-0.6 mM). A number of strategies have been employed to amplify the observed in vivo signal of MR contrast agents. Approaches include attachment of Gd(III) chelates to polymers, proteins and particles, encapsulation into micelles and caged structures, and targeting to receptors. While each of these approaches has yielded significant increases in the relaxivity of MR contrast agents (and therefore sensitivity), all of these classes of complexes possess intrinsic background signal and function solely as anatomical reporters. In order to reduce the background signal and simultaneously create probes that are modulated by biochemical processes, caged complexes were designed to coordinatively saturate the paramagnetic ion. Coupled with amplification strategies, these agents represent a means to selectively modulate the observed MR signal and function as in vivo biochemical reporters. For example, to create an in vivo MR assay of enzymatic activities and secondary messengers, agents have been designed and synthesized with removable protection groups that largely prevent access of water to a paramagnetic center. By limiting the access of bulk water (q-modulation) the unprocessed agent is designed to be an ineffective contrast agent, and hence serves as a reliable marker for regions of enzyme activity or the presence of secondary messengers. Further, we have focused on designing multimodal contrast agents that are simultaneously detectable by more than one imaging technique. For example, attaching an optical probe to a MR contrast agent provides the means to detect the probe in a whole animal and subsequently validate the results by histological methods. Finally, we describe strategies for signal amplification, and cell delivery vehicles attached to imaging probes for in vivo long-term fate mapping experiments.
Figures





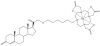



References
-
- Raymond KN, Pierre VC. Next Generation, High Relaxivity Gadolinium MRI Agents. Bioconjugate Chem. 2005;16:3–8. - PubMed
-
- Lauterbur PC. Image Formation by Incduced Local Interactions: Examples Employing Nuclear Paramagnetic Resonance. Nature. 1973;242:190–191. - PubMed
-
- Merbach A, Toth E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. John Wiley & Sons, Ltd.; New York: 2001.
-
- Caravan P, Ellison JJ, McMurry TJ, Laufer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999;99:2293–2352. - PubMed
-
- Solomon I. Relaxation processes in a system of two spins. Physics Reviews. 1955;99:559–565.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

