Light chain amyloidosis - current findings and future prospects
- PMID: 19538145
- PMCID: PMC3898330
- DOI: 10.2174/138920309789351949
Light chain amyloidosis - current findings and future prospects
Abstract
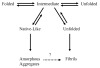
Systemic light chain amyloidosis (AL) is one of several protein misfolding diseases and is characterized by extracellular deposition of immunoglobulin light chains in the form of amyloid fibrils [1]. Immunoglobulin (Ig) proteins consist of two light chains (LCs) and two heavy chains (HCs) that ordinarily form a heterotetramer which is secreted by a plasma cell. In AL, however, a monoclonal plasma cell population produces an abundance of a pathogenic LC protein. In this case, not all of the LCs pair with the HCs, and free LCs are secreted into circulation. The LC-HC dimer is very stable, and losing this interaction may result in an unstable LC protein [2]. Additionally, somatic mutations are thought to cause amyloidogenic proteins to be less stable compared to non-amyloidogenic proteins [3-5], leading to protein misfolding and amyloid fibril formation. The amyloid fibrils cause tissue damage and cell death, leading to patient death within 12-18 months if left untreated [6]. Current therapies are harsh and not curative, including chemotherapy and autologous stem cell transplants. Studies of protein pathogenesis and fibril formation mechanisms may lead to better therapies with an improved outlook for patient survival. Much has been done to determine the molecular factors that make a particular LC protein amyloidogenic and to elucidate the mechanism of amyloid fibril formation. Anthony Fink's work, particularly with discerning the role of intermediates in the fibril formation pathway, has made a remarkable impact in the field of amyloidosis research. This review provides a general overview of the current state of AL research and also attempts to capture the most recent ideas and knowledge generated from the Fink laboratory.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
