Three decades of the class A beta-lactamase acyl-enzyme
- PMID: 19538154
- PMCID: PMC6902449
- DOI: 10.2174/138920309789351967
Three decades of the class A beta-lactamase acyl-enzyme
Abstract
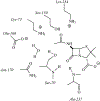
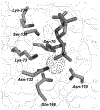
The discovery that the mechanism of beta-lactam hydrolysis catalyzed by the class A (active site serine-dependent) beta-lactamases proceeds via an acyl-enzyme intermediate was made thirty years ago. Since this discovery, the active site circumstance that enables acylation of the active site serine and further enables hydrolytic deacylation of the acyl-serine intermediate, has received extraordinary scrutiny. The justification for this scrutiny is the direct relevance of the beta-lactamases to the manifestation of bacterial resistance to the beta-lactam antibiotics, and the subsequent (to the discovery of the beta-lactamase acyl-enzyme) recognition of the direct evolutionary relationship between the serine beta-lactamase acyl-enzyme, and the penicillin binding protein acyl-enzyme that is key to beta-lactam antibiotic activity. This short review describes the early events leading to the recognition that serine beta-lactamase catalysis proceeds via an acyl-enzyme intermediate, and summarizes several of the key mechanistic studies--including infrared spectroscopy, cryoenzymology, beta-lactam design, and x-ray crystallography--that have been exploited to understand this pivotal catalytic intermediate.
Figures
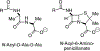

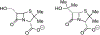
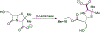
References
-
- Kresge N; Simoni RD; Hill RL Selman Waksman: the Father of Antibiotics. J. Biol. Chem, 2005, 279, e7–e8.
-
- Lee B Conformation of penicillin as a transition-state analog of the substrate of peptidoglycan transpeptidase. J. Mol. Biol, 1971, 61, 463–469. - PubMed
-
- Pratt RF Functional evolution of the serine β-lactamase active site. J. Chem. Soc., Perkin Trans. I, 2002, 2, 851–861.
-
- Kresge N; Simoni RD; Hill RL Penicillin binding in bacteria: the work of jack l. strominger. J. Biol. Chem, 2007, 282, e25–e27.
-
- Abraham E Selective reminiscences of β-lactam antibiotics: early research on penicillin and cephalosporins. Bioessays, 1990, 12, 601–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
