The contact allergen nickel sensitizes primary human endothelial cells and keratinocytes to TRAIL-mediated apoptosis
- PMID: 19538462
- PMCID: PMC3829037
- DOI: 10.1111/j.1582-4934.2009.00823.x
The contact allergen nickel sensitizes primary human endothelial cells and keratinocytes to TRAIL-mediated apoptosis
Abstract
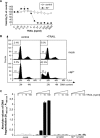
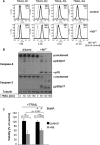
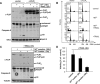
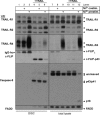
Primary endothelial cells are fully resistant to TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. Here, we demonstrate that certain environmental conditions, such as exposure to the widespread allergen nickel, can dramatically increase the susceptibility of naturally resistant primary endothelial cells or keratinocytes to TRAIL-induced apoptosis. While nickel treatment increased surface expression of the apoptosis-inducing TRAIL receptors TRAIL-R1 and TRAIL-R2, it also up-regulated the apoptosis-deficient TRAIL-R4, suggesting that modulation of TRAIL receptor expression alone is unlikely to fully account for the dramatic sensitization effect of nickel. Further analysis of candidate mediators revealed that nickel strongly repressed c-FLIP at mRNA and protein levels. Accordingly, increased activation of Caspase-8 and Caspase-3 following nickel treatment was observed. Importantly, depletion of c-FLIP by RNA interference could largely recapitulate the effect of nickel and sensitize endothelial cells to TRAIL-dependent apoptosis in the absence of nickel pre-treatment. Conversely, ectopic expression of c-FLIP(L) largely protected nickel-treated cells from TRAIL-mediated apoptosis. Our data demonstrate that one key mechanism of sensitization of primary human endothelial cells or keratinocytes is transcriptional down-regulation of c-FLIP. We hypothesize that environmental factors, exemplified by the contact allergen nickel, strongly modulate death ligand sensitivity of endothelial cells and keratinocytes thus influencing vascular and epidermal function and integrity under physiological and pathophysiological conditions.
Figures



Similar articles
-
Regulation of tumor necrosis factor-related apoptosis-inducing ligand sensitivity in primary and transformed human keratinocytes.Cancer Res. 2000 Feb 1;60(3):553-9. Cancer Res. 2000. PMID: 10676636
-
Selective inhibition of FLICE-like inhibitory protein expression with small interfering RNA oligonucleotides is sufficient to sensitize tumor cells for TRAIL-induced apoptosis.Mol Med. 2002 Nov;8(11):725-32. Mol Med. 2002. PMID: 12520089 Free PMC article.
-
Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells.Mol Cancer Ther. 2008 Sep;7(9):2649-61. doi: 10.1158/1535-7163.MCT-08-0148. Mol Cancer Ther. 2008. PMID: 18790747
-
Suppression of cFLIP is sufficient to sensitize human melanoma cells to TRAIL- and CD95L-mediated apoptosis.Oncogene. 2008 May 15;27(22):3211-20. doi: 10.1038/sj.onc.1210985. Epub 2007 Dec 17. Oncogene. 2008. PMID: 18084329
-
Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL.J Cell Physiol. 2018 Oct;233(10):6470-6485. doi: 10.1002/jcp.26585. Epub 2018 May 9. J Cell Physiol. 2018. PMID: 29741767 Review.
Cited by
-
Nickel allergies: paying the Toll for innate immunity.J Mol Med (Berl). 2011 Oct;89(10):961-70. doi: 10.1007/s00109-011-0780-0. Epub 2011 Jun 23. J Mol Med (Berl). 2011. PMID: 21698426 Review.
-
Allergic contact dermatitis in psoriasis patients: typical, delayed, and non-interacting.PLoS One. 2014 Jul 24;9(7):e101814. doi: 10.1371/journal.pone.0101814. eCollection 2014. PLoS One. 2014. PMID: 25058585 Free PMC article.
-
Epidermal RelA specifically restricts contact allergen-induced inflammation and apoptosis in skin.J Invest Dermatol. 2014 Oct;134(10):2541-2550. doi: 10.1038/jid.2014.193. Epub 2014 Apr 16. J Invest Dermatol. 2014. PMID: 24739902
-
Animal models for nickel allergy.Nat Nanotechnol. 2011 Sep 6;6(9):533; author reply 533. doi: 10.1038/nnano.2011.143. Nat Nanotechnol. 2011. PMID: 21897378 No abstract available.
-
Adaptation in the innate immune system and heterologous innate immunity.Cell Mol Life Sci. 2014 Nov;71(21):4115-30. doi: 10.1007/s00018-014-1676-2. Epub 2014 Jul 6. Cell Mol Life Sci. 2014. PMID: 24997561 Free PMC article. Review.
References
-
- Krob HA, Fleischer AB, Jr, D’Agostino R, Jr, et al. Prevalence and relevance of contact dermatitis allergens: a meta-analysis of 15 years of published T.R.U.E. test data. J Am Acad Dermatol. 2004;51:349–53. - PubMed
-
- Larese F, Gianpietro A, Venier M, et al. In vitro percutaneous absorption of metal compounds. Toxicol Lett. 2007;170:49–56. - PubMed
-
- Koster R, Vieluf D, Kiehn M, et al. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet. 2000;356:1895–7. - PubMed
-
- Hostynek JJ. Sensitization to nickel: etiology, epidemiology, immune reactions, prevention, and therapy. Rev Environ Health. 2006;21:253–80. - PubMed
-
- Goebeler M, Roth J, Brocker EB, et al. Activation of nuclear factor-kappa B and gene expression in human endothelial cells by the common haptens nickel and cobalt. J Immunol. 1995;155:2459–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

