The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment
- PMID: 19538929
- PMCID: PMC2768135
- DOI: 10.1016/j.immuni.2009.04.014
The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment
Abstract
Effector T cell differentiation requires the simultaneous integration of multiple, and sometimes opposing, cytokine signals. We demonstrated mTOR's role in dictating the outcome of T cell fate. mTOR-deficient T cells displayed normal activation and IL-2 production upon initial stimulation. However, such cells failed to differentiate into T helper 1 (Th1), Th2, or Th17 effector cells. The inability to differentiate was associated with decreased STAT transcription factor activation and failure to upregulate lineage-specific transcription factors. Under normally activating conditions, T cells lacking mTOR differentiated into Foxp3(+) regulatory T cells. This was associated with hyperactive Smad3 activation in the absence of exogenous TGF-beta. Surprisingly, T cells selectively deficient in TORC1 do not divert to a regulatory T cell pathway, implicating both TORC1 and TORC2 in preventing the generation of regulatory T cells. Overall, our studies suggest that mTOR kinase signaling regulates decisions between effector and regulatory T cell lineage commitment.
Figures

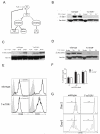
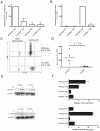
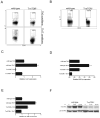


Comment in
-
m(en)TOR(ing) differentiating T helper cells.Immunity. 2009 Jun 19;30(6):759-61. doi: 10.1016/j.immuni.2009.06.002. Immunity. 2009. PMID: 19538925
References
-
- Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. - PubMed
-
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. - PubMed
-
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

