Compression regulates mitotic spindle length by a mechanochemical switch at the poles
- PMID: 19540117
- PMCID: PMC2722833
- DOI: 10.1016/j.cub.2009.05.056
Compression regulates mitotic spindle length by a mechanochemical switch at the poles
Abstract
Background: Although the molecules involved in mitosis are becoming better characterized, we still lack an understanding of the emergent mechanical properties of the mitotic spindle. For example, we cannot explain how spindle length is determined. To gain insight into how forces are generated and responded to in the spindle, we developed a method to apply controlled mechanical compression to metaphase mitotic spindles in living mammalian cells while monitoring microtubules and kinetochores by fluorescence microscopy.
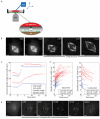
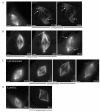
Results: Compression caused reversible spindle widening and lengthening to a new steady state. Widening was a passive mechanical response, and lengthening was an active mechanochemical process requiring microtubule polymerization but not kinesin-5 activity. Spindle morphology during lengthening and drug perturbations suggested that kinetochore fibers are pushed outward by pole-directed forces generated within the spindle. Lengthening of kinetochore fibers occurred by inhibition of microtubule depolymerization at poles, with no change in sliding velocity, interkinetochore stretching, or kinetochore dynamics.
Conclusions: We propose that spindle length is controlled by a mechanochemical switch at the poles that regulates the depolymerization rate of kinetochore fibers in response to compression and discuss models for how this switch is controlled. Poleward force appears to be exerted along kinetochore fibers by some mechanism other than kinesin-5 activity, and we speculate that it may arise from polymerization pressure from growing plus ends of interpolar microtubules whose minus ends are anchored in the fiber. These insights provide a framework for conceptualizing mechanical integration within the spindle.
Figures



References
-
- Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. - PubMed
-
- Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407:41–47. - PubMed
-
- Pickett-Heaps JD, Forer A, Spurck T. Traction fibre: toward a “tensegral” model of the spindle. Cell Motility and the Cytoskeleton. 1997;37:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

