Requirement of TLR4 and CD14 in dendritic cell activation by Hemagglutinin B from Porphyromonas gingivalis
- PMID: 19540594
- PMCID: PMC2763909
- DOI: 10.1016/j.molimm.2009.05.022
Requirement of TLR4 and CD14 in dendritic cell activation by Hemagglutinin B from Porphyromonas gingivalis
Abstract
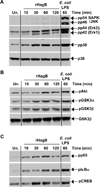
Porphyromonas gingivalis is a Gram-negative anaerobic bacterium that is one of the causative agents of chronic adult periodontal disease. Among the potential virulence factors of P. gingivalis are the hemagglutinins. Recombinant Hemagglutinin B (rHagB) from P. gingivalis has been shown to activate the immune system by inducing specific antibodies that protect against experimental periodontal bone loss following P. gingivalis infection. Since different microbial products can stimulate dendritic cells (DC) through Toll-like receptors (TLRs), subsequently leading to T cell activation and antibody production, we wanted to investigate the immunostimulatory effect of rHagB on DC and the role of TLR signaling in this process. Using an endotoxin free rHagB preparation, our results show that stimulation of murine bone marrow-derived DC with rHagB leads to upregulation of the costimulatory molecules CD86 and CD40, activation of p38 and ERK MAP kinases, transcription factors NF-kappaB, CREB and IRF-3 and the production of IL-6, TNF-alpha, IL-12p40 and to a lesser extent IL-10 and IFN-beta. This activation process was absolutely dependent on TLR4 and CD14. While upregulation of CD86 was independent of the adaptor molecule MyD88, CD40 upregulation and optimal cytokine (IL-6, TNF-alpha, IL-12p40, IL-10 and IFN-beta) production required both MyD88 and TRIF molecules. These results are of importance since they are the first to provide insights into the interaction of rHagB with DC and TLRs. The information from this study will aid in the design of effective vaccines strategies against chronic adult periodontal disease.
Figures







References
-
- Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. - PubMed
-
- Argueta-Donohue J, Carrillo N, Valdes-Reyes L, Zentella A, Aguirre-Garcia M, Becker I, Gutierrez-Kobeh L. Leishmania mexicana: participation of NF-kappaB in the differential production of IL-12 in dendritic cells and monocytes induced by lipophosphoglycan (LPG) Exp. Parasitol. 2008;120:1–9. - PubMed
-
- Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 2001;166:3837–3845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

