The influence of the membrane on neurosteroid actions at GABA(A) receptors
- PMID: 19541427
- PMCID: PMC2794963
- DOI: 10.1016/j.psyneuen.2009.05.020
The influence of the membrane on neurosteroid actions at GABA(A) receptors
Abstract
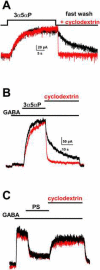
Modern views of anesthetic neurosteroid interaction with the GABA(A) receptor conceptualize steroid ligands interacting with a protein binding site on the receptor. It has generally been assumed that the steroid interaction/binding site is contained in an extracellular domain of the receptor, and that steroid interactions are of high potency, evidenced by the low aqueous ligand concentrations required to achieve potentiation of channel function. We have been considering implications of the observations that steroids are quite lipophilic and that recently identified putative steroid binding sites are in transmembrane domains of the receptor. Accordingly, we expect that both the effective plasma membrane steroid concentration and steroid pharmacophore properties will contribute to steady-state potency and to the lifetime of steroid actions following removal of free aqueous steroid. Here we review our recent studies that address the evidence that membrane partitioning and intracellular accumulation are non-specific contributors to the effects of anesthetic steroids at GABA(A) receptors. We compare and contrast the profile of anesthetic steroids with that of sulfated steroids that negatively regulate GABA(A) receptor function. These studies give rise to the view that the inherent affinity of anesthetic steroid for GABA(A) receptors is very low; low effective aqueous concentrations are accounted for by lipid partitioning. This yields a very different picture of the interaction of neurosteroids with the GABA(A) receptor than that of steroid interactions with classical intracellular steroid receptors, which exhibit inherently high affinity. These considerations have practical implications for actions of endogenous neurosteroids. Lipophilicity will tend to promote autocrine actions of neurosteroids at GABA(A) receptors within cells that synthesize neurosteroids, and lipophilic retention will limit intercellular diffusion from the source of steroid synthesis. Lipophilicity and steroid access to the receptor binding sites also must be considerations in drug design if drugs are to effectively reach the target GABA(A) receptor site.
Figures


References
-
- Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, Palin R, Prosser A, Rees DC, Rosair GM, Stevenson D, Tarver GJ, Zhang MQ. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure-activity relationships. J. Med. Chem. 2002;45:1806–1816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

