Transcription coactivator SAYP combines chromatin remodeler Brahma and transcription initiation factor TFIID into a single supercomplex
- PMID: 19541607
- PMCID: PMC2708721
- DOI: 10.1073/pnas.0901801106
Transcription coactivator SAYP combines chromatin remodeler Brahma and transcription initiation factor TFIID into a single supercomplex
Abstract
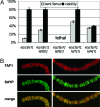
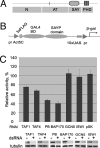
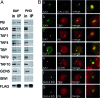
Transcription activation by RNA polymerase II is a complicated process driven by combined, precisely coordinated action of a wide array of coactivator complexes, which carry out chromatin-directed activities and nucleate the assembly of the preinitiation complex on the promoter. Using various techniques, we have shown the existence of a stable coactivator supercomplex consisting of the chromatin-remodeling factor Brahma (SWI/SNF) and the transcription initiation factor TFIID, named BTFly (Brahma and TFIID in one assembly). The coupling of Brahma and TFIID is mediated by the SAYP factor, whose evolutionarily conserved activation domain SAY can directly bind to both BAP170 subunit of Brahma and TAF5 subunit of TFIID. The integrity of BTFly is crucial for its ability to activate transcription. BTFly is distributed genome-wide and appears to be a means of effective transcription activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


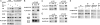
Similar articles
-
[Novel conservative domain of SAYP coactivator mediates TFIID and Brahma transcriptional complexes interaction].Mol Biol (Mosk). 2010 Sep-Oct;44(5):867-75. Mol Biol (Mosk). 2010. PMID: 21090241 Russian.
-
[Novel complex, formed by transcription coactivator SAYP].Mol Biol (Mosk). 2009 Nov-Dec;43(6):1055-62. Mol Biol (Mosk). 2009. PMID: 20088382 Russian.
-
[SAYP--a novel regulator of metazoan development].Genetika. 2010 Aug;46(8):1033-40. Genetika. 2010. PMID: 20873199 Russian.
-
SAGA and TFIID: Friends of TBP drifting apart.Biochim Biophys Acta Gene Regul Mech. 2021 Feb;1864(2):194604. doi: 10.1016/j.bbagrm.2020.194604. Epub 2020 Jul 14. Biochim Biophys Acta Gene Regul Mech. 2021. PMID: 32673655 Review.
-
Zooming in on Transcription Preinitiation.J Mol Biol. 2016 Jun 19;428(12):2581-2591. doi: 10.1016/j.jmb.2016.04.003. Epub 2016 Apr 8. J Mol Biol. 2016. PMID: 27067110 Free PMC article. Review.
Cited by
-
Insulator protein Su(Hw) recruits SAGA and Brahma complexes and constitutes part of Origin Recognition Complex-binding sites in the Drosophila genome.Nucleic Acids Res. 2013 Jun;41(11):5717-30. doi: 10.1093/nar/gkt297. Epub 2013 Apr 22. Nucleic Acids Res. 2013. PMID: 23609538 Free PMC article.
-
PHF10 subunit of PBAF complex mediates transcriptional activation by MYC.Oncogene. 2021 Oct;40(42):6071-6080. doi: 10.1038/s41388-021-01994-0. Epub 2021 Aug 31. Oncogene. 2021. PMID: 34465901 Free PMC article.
-
SS18 together with animal-specific factors defines human BAF-type SWI/SNF complexes.PLoS One. 2012;7(3):e33834. doi: 10.1371/journal.pone.0033834. Epub 2012 Mar 19. PLoS One. 2012. PMID: 22442726 Free PMC article.
-
Paip2 is localized to active promoters and loaded onto nascent mRNA in Drosophila.Cell Cycle. 2018;17(14):1708-1720. doi: 10.1080/15384101.2018.1496738. Epub 2018 Aug 1. Cell Cycle. 2018. PMID: 29995569 Free PMC article.
-
Polycomb and Trithorax Group Proteins: The Long Road from Mutations in Drosophila to Use in Medicine.Acta Naturae. 2020 Oct-Dec;12(4):66-85. doi: 10.32607/actanaturae.11090. Acta Naturae. 2020. PMID: 33456979 Free PMC article.
References
-
- Naar AM, Lemon BD, Tjian R. Transcriptional coactivator complexes. Annu Rev Biochem. 2001;70:475–501. - PubMed
-
- Taatjes DJ, Marr MT, Tjian R. Regulatory diversity among metazoan co-activator complexes. Nat Rev Mol Cell Biol. 2004;5:403–410. - PubMed
-
- Mohrmann L, Verrijzer CP. Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim Biophys Acta - Gene Structure and Expression. 2005;1681:59–73. - PubMed
-
- Neely KE, Workman JL. The complexity of chromatin remodeling and its links to cancer. Biochim Biophys Acta. 2002;1603:19–29. - PubMed
-
- Albright SR, Tjian R. TAFs revisited: More data reveal new twists and confirm old ideas. Gene. 2000;242:1–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

