An icosahedral algal virus has a complex unique vertex decorated by a spike
- PMID: 19541619
- PMCID: PMC2708736
- DOI: 10.1073/pnas.0904716106
An icosahedral algal virus has a complex unique vertex decorated by a spike
Abstract
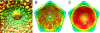
Paramecium bursaria Chlorella virus-1 is an icosahedrally shaped, 1,900-A-diameter virus that infects unicellular eukaryotic green algae. A 5-fold symmetric, 3D reconstruction using cryoelectron microscopy images has now shown that the quasiicosahedral virus has a unique vertex, with a pocket on the inside and a spike structure on the outside of the capsid. The pocket might contain enzymes for use in the initial stages of infection. The unique vertex consists of virally coded proteins, some of which have been identified. Comparison of shape, size, and location of the spike with similar features in bacteriophages T4 and P22 suggests that the spike might be a cell-puncturing device. Similar asymmetric features may have been missed in previous analyses of many other viruses that had been assumed to be perfectly icosahedral.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dunigan DD, Fitzgerald LA, Van Etten JL. Phycodnaviruses: A peek at genetic diversity. Virus Res. 2006;117:119–132. - PubMed
-
- Meints RH, Lee K, Burbank DE, Van Etten JL. Infection of a Chlorella-like alga with the virus, PBCV-1: Ultrastructural studies. Virology. 1984;138:341–346. - PubMed
-
- Meints RH, Burbank DE, Van Etten JL, Lamport DT. Properties of the Chlorella receptor for the virus PBCV-1. Virology. 1988;164:15–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

