Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats
- PMID: 19541621
- PMCID: PMC2698887
- DOI: 10.1073/pnas.0901176106
Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats
Abstract
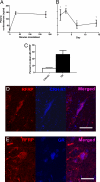
The subjective experience of stress leads to reproductive dysfunction in many species, including rodents and humans. Stress effects on reproduction result from multilevel interactions between the hormonal stress response system, i.e., the hypothalamic-pituitary-adrenal (HPA) axis, and the hormonal reproductive system, i.e., the hypothalamic-pituitary-gonadal (HPG) axis. A novel negative regulator of the HPG axis known as gonadotropin-inhibitory hormone (GnIH) was recently discovered in quail, and orthologous neuropeptides known as RFamide-related peptides (RFRPs) have also been identified in rodents and primates. It is currently unknown, however, whether GnIH/RFRPs influence HPG axis activity in response to stress. We show here that both acute and chronic immobilization stress lead to an up-regulation of RFRP expression in the dorsomedial hypothalamus (DMH) of adult male rats and that this increase in RFRP is associated with inhibition of downstream HPG activity. We also show that adrenalectomy blocks the stress-induced increase in RFRP expression. Immunohistochemistry revealed that 53% of RFRP cells express receptors for glucocorticoids (GCs), indicating that adrenal GCs can mediate the stress effect through direct action on RFRP cells. It is thought that stress effects on central control of reproduction are largely mediated by direct or indirect effects on GnRH-secreting neurons. Our data show that stress-induced increases in adrenal GCs cause an increase in RFRP that contributes to hypothalamic suppression of reproductive function. This novel insight into HPA-HPG interaction provides a paradigm shift for work on stress-related reproductive dysfunction and infertility, and indicates that future work on stress and reproductive system interactions must include investigation of the role of GnIH/RFRP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gondal axis: Peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. - PubMed
-
- Rivier C, Rivier J, Vale W. Stress-induced inhibition of reproductive functions: Role of endogenous corticotropin-releasing factor. Science. 1986;231:607–609. - PubMed
-
- Du Ruisseau P, Tache Y, Brazeau P, Collu R. Effects of chronic immobilization stress on pituitary hormone secretion, on hypothalamic factor levels, and on pituitary responsiveness to LHRH and TRH in female rats. Neuroendocrinology. 1979;29:90–99. - PubMed
-
- Gonzalez-Quijano MI, Ariznavarreta C, Martin AI, Treguerres JAF, Lopez-Calderon A. Naltrexone does not reverse the inhibitory effect of chronic restraint on gonadotropin secretion in the intact male rat. Neuroendocrinology. 1991;54:447–453. - PubMed
-
- Sato Y, et al. Effects of long-term psychological stress on sexual behavior and brain catecholamine levels. J Androl. 1996;17:83–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

