Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues
- PMID: 19541627
- PMCID: PMC2700905
- DOI: 10.1073/pnas.0900174106
Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues
Abstract
Physical forces generated by cells drive morphologic changes during development and can feedback to regulate cellular phenotypes. Because these phenomena typically occur within a 3-dimensional (3D) matrix in vivo, we used microelectromechanical systems (MEMS) technology to generate arrays of microtissues consisting of cells encapsulated within 3D micropatterned matrices. Microcantilevers were used to simultaneously constrain the remodeling of a collagen gel and to report forces generated during this process. By concurrently measuring forces and observing matrix remodeling at cellular length scales, we report an initial correlation and later decoupling between cellular contractile forces and changes in tissue morphology. Independently varying the mechanical stiffness of the cantilevers and collagen matrix revealed that cellular forces increased with boundary or matrix rigidity whereas levels of cytoskeletal and extracellular matrix (ECM) proteins correlated with levels of mechanical stress. By mapping these relationships between cellular and matrix mechanics, cellular forces, and protein expression onto a bio-chemo-mechanical model of microtissue contractility, we demonstrate how intratissue gradients of mechanical stress can emerge from collective cellular contractility and finally, how such gradients can be used to engineer protein composition and organization within a 3D tissue. Together, these findings highlight a complex and dynamic relationship between cellular forces, ECM remodeling, and cellular phenotype and describe a system to study and apply this relationship within engineered 3D microtissues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

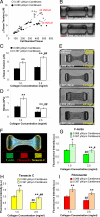

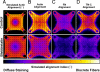
References
-
- Beloussov LV, Dorfman JG, Cherdantzev VG. Mechanical stresses and morphological patterns in amphibian embryos. J Embryol Exp Morphol. 1975;34:559–574. - PubMed
-
- Taber LA. Biomechanics of growth, remodeling, and morphogenesis. Appl Mech Rev. 1995;48:487–545.
-
- Keller R, Davidson LA, Shook DR. How we are shaped: The biomechanics of gastrulation. Differentiation. 2003;71:171–205. - PubMed
-
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. - PubMed
-
- Wolff J. In: Das gesetz der transformation der knochen, Kirschwald. Maquet P, Furlong R, translators. Berlin: Springer; 1892. (1986) (German)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

