Real-time molecular monitoring of chemical environment in obligate anaerobes during oxygen adaptive response
- PMID: 19541631
- PMCID: PMC2697112
- DOI: 10.1073/pnas.0902070106
Real-time molecular monitoring of chemical environment in obligate anaerobes during oxygen adaptive response
Abstract
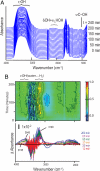
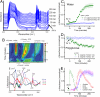
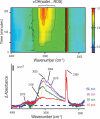
Determining the transient chemical properties of the intracellular environment can elucidate the paths through which a biological system adapts to changes in its environment, for example, the mechanisms that enable some obligate anaerobic bacteria to survive a sudden exposure to oxygen. Here we used high-resolution Fourier transform infrared (FTIR) spectromicroscopy to continuously follow cellular chemistry within living obligate anaerobes by monitoring hydrogen bond structures in their cellular water. We observed a sequence of well orchestrated molecular events that correspond to changes in cellular processes in those cells that survive, but only accumulation of radicals in those that do not. We thereby can interpret the adaptive response in terms of transient intracellular chemistry and link it to oxygen stress and survival. This ability to monitor chemical changes at the molecular level can yield important insights into a wide range of adaptive responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

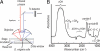

References
-
- Cypionka H. Oxygen respiration by Desulfovibrio species. Annu Rev Microbiol. 2000;54:827–848. - PubMed
-
- Baughn AD, Malamy MH. Molecular Basis for Aerotolerance of the Obligately Anaerobic Bacteroides Spp. In: Nakano MM, Zuber P, editors. Strict and Facultative Anaerobes: Medical and Environmental Aspects. New York: CRC Press; 2004. pp. 161–169.
-
- Dolla A, Fournier M, Dermoun Z. Oxygen defense in sulfate-reducing bacteria. J Biotechnol. 2006;126:87–100. - PubMed

