Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum
- PMID: 19541636
- PMCID: PMC2697114
- DOI: 10.1073/pnas.0903408106
Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum
Abstract
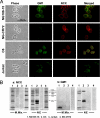
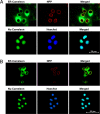
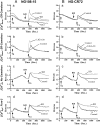
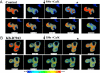
The inner membrane of the nuclear envelope (NE) was previously shown to contain a Na/Ca exchanger (NCX) tightly linked to GM1 ganglioside that mediates transfer of nucleoplasmic Ca(2+) to the NE lumen and constitutes a cytoprotective mechanism. This transfer was initially observed with isolated nuclei and is now demonstrated in living cells in relation to subcellular Ca(2+) dynamics. Four cell lines with varying expression of NCX and GM1 in the NE were transfected with cameleon-fluorescent Ca(2+) indicators genetically targeted to NE/endoplasmic reticulum (ER) and nucleoplasm to monitor [Ca(2+)](ne/er) and [Ca(2+)](n) respectively. Cytosolic Ca(2+) ([Ca(2+)](cyt)) was indicated with fura-2. Thapsigargin caused progressive loss of [Ca(2+)](ne/er), which was rapidly replaced on addition of extrinsic Ca(2+) to those cells containing fully functional NCX/GM1: differentiated NG108-15 and C6 cells. Reduced elevation of [Ca(2+)](ne/er) following thapsigargin depletion occurred in cells containing little or no GM1 in the NE: undifferentiated NG108-15 and NG-CR72 cells. No change in [Ca(2+)](ne/er) due to applied Ca(2+) was seen in Jurkat cells, which entirely lack NCX. Ca(2+) entry to NE/ER was also blocked by KB-R7943, inhibitor of NCX. [Ca(2+)](n) and [Ca(2+)](cyt) were elevated independent of [Ca(2+)](ne/er) and remained in approximate equilibrium with each other. Ca(2+) rise in the ER originated in the NE region and extended to the entire ER network. These results indicate the nuclear NCX/GM1 complex acts to gate Ca(2+) transfer from cytosol to ER, an alternate route to the sarcoplasmic/endoplasmic reticulum calcium ATPase pump. They also suggest a possible contributory mechanism for independent regulation of nuclear Ca(2+).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
New findings on nuclear gangliosides: overview on metabolism and function.J Neurochem. 2011 Mar;116(5):714-20. doi: 10.1111/j.1471-4159.2010.07115.x. Epub 2011 Jan 13. J Neurochem. 2011. PMID: 21214576 Review.
-
C6 cells express a sodium-calcium exchanger/GM1 complex in the nuclear envelope but have no exchanger in the plasma membrane: comparison to astrocytes.J Neurosci Res. 2004 May 1;76(3):363-75. doi: 10.1002/jnr.20068. J Neurosci Res. 2004. PMID: 15079865
-
Presence of sodium-calcium exchanger/GM1 complex in the nuclear envelope of non-neural cells: nature of exchanger-GM1 interaction.Neurochem Res. 2004 Nov;29(11):2135-46. doi: 10.1007/s11064-004-6887-8. Neurochem Res. 2004. PMID: 15662848
-
GM1 ganglioside: another nuclear lipid that modulates nuclear calcium. GM1 potentiates the nuclear sodium-calcium exchanger.Can J Physiol Pharmacol. 2006 Mar-Apr;84(3-4):393-402. doi: 10.1139/y05-133. Can J Physiol Pharmacol. 2006. PMID: 16902585 Review.
-
Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside.J Neurochem. 2002 Jun;81(6):1185-95. doi: 10.1046/j.1471-4159.2002.00917.x. J Neurochem. 2002. PMID: 12068067
Cited by
-
Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment.Biomolecules. 2019 Sep 20;9(10):513. doi: 10.3390/biom9100513. Biomolecules. 2019. PMID: 31547139 Free PMC article. Review.
-
Nuclear lipid mediators: Role of nuclear sphingolipids and sphingosine-1-phosphate signaling in epigenetic regulation of inflammation and gene expression.J Cell Biochem. 2018 Aug;119(8):6337-6353. doi: 10.1002/jcb.26707. Epub 2018 May 8. J Cell Biochem. 2018. PMID: 29377310 Free PMC article. Review.
-
Nuclear Na+/K+-ATPase plays an active role in nucleoplasmic Ca2+ homeostasis.J Cell Sci. 2012 Dec 15;125(Pt 24):6137-47. doi: 10.1242/jcs.114959. Epub 2012 Oct 17. J Cell Sci. 2012. PMID: 23077175 Free PMC article.
-
Intranasal infusion of GD3 and GM1 gangliosides downregulates alpha-synuclein and controls tyrosine hydroxylase gene in a PD model mouse.Mol Ther. 2021 Oct 6;29(10):3059-3071. doi: 10.1016/j.ymthe.2021.06.005. Epub 2021 Jun 8. Mol Ther. 2021. PMID: 34111562 Free PMC article.
-
A new cell-penetrating peptide that blocks the autoinhibitory XIP domain of NCX1 and enhances antiporter activity.Mol Ther. 2015 Mar;23(3):465-76. doi: 10.1038/mt.2014.231. Epub 2014 Dec 11. Mol Ther. 2015. PMID: 25582710 Free PMC article.
References
-
- Petersen OH, Gerasimenko OV, Gerasimenko JV, Mogami H, Tepikin AV. The calcium store in the nuclear envelope. Cell Calcium. 1998;23:87–90. - PubMed
-
- Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP- ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–444. - PubMed
-
- Koppler P, Matter N, Malviya AN. Evidence for stereospecific inositol 1,3,4,5-[3H]tetrakisphosphate binding sites on rat liver nuclei. J Biol Chem. 1993;268:26248–26252. - PubMed
-
- Stehno-Bittel L, Lückhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

