Sporulation in mycobacteria
- PMID: 19541637
- PMCID: PMC2705590
- DOI: 10.1073/pnas.0904104106
Sporulation in mycobacteria
Abstract
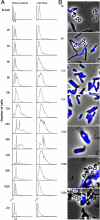
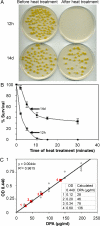
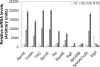
Mycobacteria owe their success as pathogens to their ability to persist for long periods within host cells in asymptomatic, latent forms before they opportunistically switch to the virulent state. The molecular mechanisms underlying the transition into dormancy and emergence from it are not clear. Here we show that old cultures of Mycobacterium marinum contained spores that, upon exposure to fresh medium, germinated into vegetative cells and reappeared again in stationary phase via endospore formation. They showed many of the usual characteristics of well-known endospores. Homologues of well-known sporulation genes of Bacillus subtilis and Streptomyces coelicolor were detected in mycobacteria genomes, some of which were verified to be transcribed during appropriate life-cycle stages. We also provide data indicating that it is likely that old Mycobacterium bovis bacillus Calmette-Guérin cultures form spores. Together, our data show sporulation as a lifestyle adapted by mycobacteria under stress and tempt us to suggest this as a possible mechanism for dormancy and/or persistent infection. If so, this might lead to new prophylactic strategies.
Conflict of interest statement
Conflict of interest statement: A patent application has been filed based on the discovery that mycobacteria form spores, with J.G., P.L., S.D., and L.A.K. listed as inventors. The cost for the patent application has been financed by MARFL AB (Sweden) where L.A.K. is a shareholder.
Figures


Similar articles
-
Do mycobacteria produce endospores?Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):878-81. doi: 10.1073/pnas.0911299107. Epub 2009 Dec 22. Proc Natl Acad Sci U S A. 2010. PMID: 20080769 Free PMC article.
-
Growth, cell division and sporulation in mycobacteria.Antonie Van Leeuwenhoek. 2010 Aug;98(2):165-77. doi: 10.1007/s10482-010-9446-0. Epub 2010 May 1. Antonie Van Leeuwenhoek. 2010. PMID: 20437098 Free PMC article.
-
Combined scanning transmission X-ray and electron microscopy for the characterization of bacterial endospores.FEMS Microbiol Lett. 2014 Sep;358(2):188-93. doi: 10.1111/1574-6968.12539. Epub 2014 Aug 12. FEMS Microbiol Lett. 2014. PMID: 25048294
-
Genetic and physiological regulation of bacterial endospore development.Pol J Microbiol. 2007;56(1):11-7. Pol J Microbiol. 2007. PMID: 17419184 Review.
-
Bacillus subtilis Systems Biology: Applications of -Omics Techniques to the Study of Endospore Formation.Microbiol Spectr. 2014 Apr;2(2). doi: 10.1128/microbiolspec.TBS-0019-2013. Microbiol Spectr. 2014. PMID: 26105826 Review.
Cited by
-
Single-cell elemental analysis of bacteria: quantitative analysis of polyphosphates in Mycobacterium tuberculosis.Front Cell Infect Microbiol. 2012 May 24;2:63. doi: 10.3389/fcimb.2012.00063. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919654 Free PMC article.
-
Pathogen quantitation in complex matrices: a multi-operator comparison of DNA extraction methods with a novel assessment of PCR inhibition.PLoS One. 2011 Mar 23;6(3):e17916. doi: 10.1371/journal.pone.0017916. PLoS One. 2011. PMID: 21448453 Free PMC article.
-
Mycobacterium tuberculosis modulates its cell surface via an oligopeptide permease (Opp) transport system.FASEB J. 2009 Dec;23(12):4091-104. doi: 10.1096/fj.09-132407. Epub 2009 Aug 11. FASEB J. 2009. PMID: 19671666 Free PMC article.
-
Growth and cell-division in extensive (XDR) and extremely drug resistant (XXDR) tuberculosis strains: transmission and atomic force observation.Int J Clin Exp Med. 2010 Sep 30;3(4):308-14. Int J Clin Exp Med. 2010. PMID: 21072265 Free PMC article.
-
New approaches in the diagnosis and treatment of latent tuberculosis infection.Respir Res. 2010 Dec 3;11(1):169. doi: 10.1186/1465-9921-11-169. Respir Res. 2010. PMID: 21126375 Free PMC article. Review.
References
-
- Duker AA, Portaels F, Hale M. Pathways of Mycobacterium ulcerans infection: A review. Environ Int. 2006;32:567–573. - PubMed
-
- Butler D. New fronts in an old war. Nature. 2000;406:670–672. - PubMed
-
- Corbett EL, et al. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

